Brain Cancer
Latest News
Latest Videos

CME Content
More News
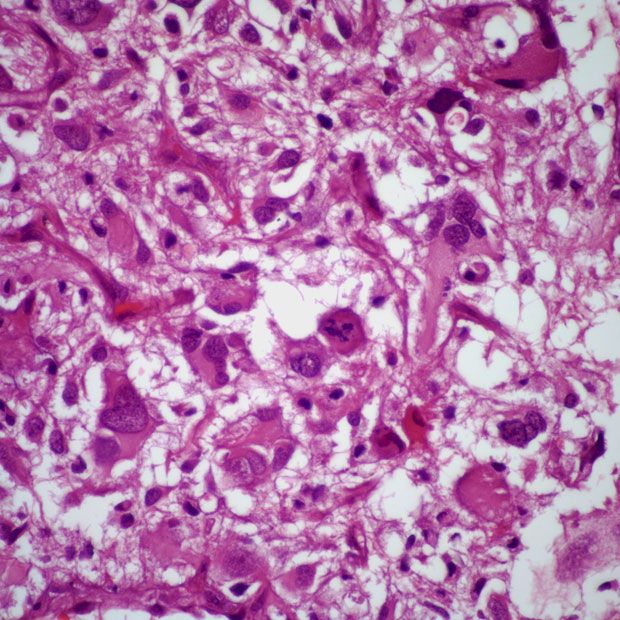
Overall survival is expected to continue improving among patients with glioblastoma being treated with frontline olaptesed pegol plus radiotherapy and bevacizumab who remain on the phase 1/2 GLORA study.

MDNA55 appears tolerable and effective in a cohort of patients enrolled in a phase 2b trial, showing sustained efficacy in subgroup analyses.
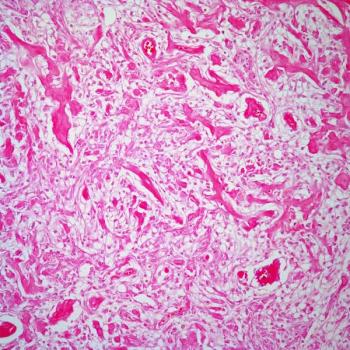
Data from a phase 2 trial assessing lenalidomide in patients under 22 years of age also highlight an optimal dose level for this population.
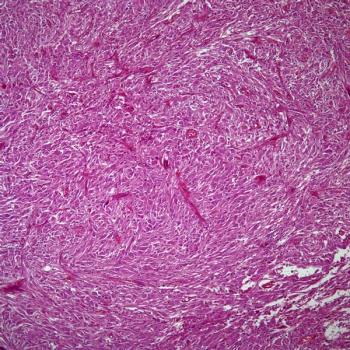
ERAS-801 is under investigation as part of the phase 1 THUNDERBBOLT-1 trial in a population of patients with EGFR-mutant, recurrent glioblastoma.
Common treatment-emergent adverse effects among patients receiving PRT811 for uveal melanoma or advanced glioma include nausea and vomiting in a phase 1 study.

Treatment with tovorafenib appears well tolerated among pediatric patients with low-grade glioma, according to findings from the phase 2 FIREFLY trial.
In the study, 331 patients with IDH-mutated low-grade glioma were randomized to receive oral vorasidenib at 40 mg once daily or matched placebo in 28-day cycles.

Data from a cohort study suggest MGMT promoter methylation could serve as a stratification factor for patients with low-grade and anaplastic gliomas harboring IDH–wild-type or IDH-mutant and co-deleted tumors.
Patients under the age of 3 with neuroblastoma experienced no significant negative impact on survival when their disease was reclassified from high-risk to intermediate-risk and their therapy was thusly reduced.

A phase 2 trial indicates that limited surgery and post-operative proton therapy result in a high rate of tumor control and less severe complications in young patients with craniopharyngioma.

Treatment with 20 mg/m2 of lenalidomide in patients under 22 years old who have pilocytic astrocytomas and optic pathway gliomas appears to be safe and effective.

Investigators of a phase 1/2 trial report that CAR T cells targeting disialoganglioside GD2 may result in long-lasting antitumor activity in young patients with high-risk neuroblastoma.

Investigators will assess INB-400 as a treatment for patients with newly diagnosed glioblastoma in a phase 2 trial expected to initiate in the second half of 2023.
ERAS-801, which now has FDA fast track designation for glioblastoma with EGFR alterations, is currently under investigation as a monotherapy in the phase 1 THUNDERBBOLT-1 trial.

Javier Orozco-Mera, MD, FACS, MSc, discusses signaling pathways and molecular mechanisms involved in the relapse of glioblastoma.
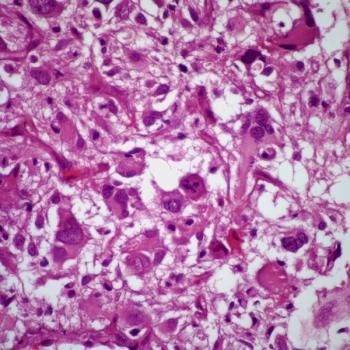
Investigators are assessing tirabrutinib, which received orphan drug designation from the FDA, as a treatment for patients with relapsed or refractory primary central nervous system lymphoma in the phase 2 PROSPECT study.
Javier Orozco-Mera, MD, FACS, MSc, and colleagues assess the signaling pathways and molecular mechanisms involved in the relapse of glioblastoma.

Investigators are assessing FORE8394, which received orphan drug designation from the FDA, in primary brain and central nervous system malignancies in the phase 2 FORTE trial.

Results from the phase 2 CDRB436G2201 trial lead to the approval of dabrafenib plus trametinib in patients with low-grade glioma and a BRAF V600E mutation who require systemic therapy.
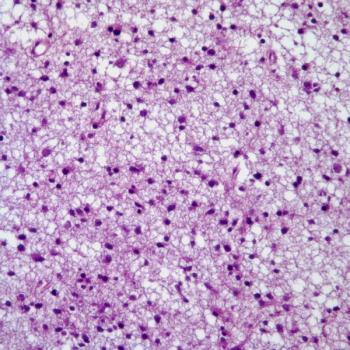
Vorasidenib monotherapy appears to improve clinical outcomes among patients with IDH-mutant low-grade glioma, meeting 2 end points in the phase 3 INDIGO trial.
The FDA gives orphan drug designation to BEA-17, an investigational small molecule degrader that may benefit those with a common and aggressive brain tumor.

Azeliragon was first evaluated in a group of patients with Alzheimer’s disease prior to being assessed in patients with glioblastoma, a fast-growing and aggressive brain tumor.
Data from the phase 2 FIREFLY-1 trial indicated that tovorafenib yielded responses among pretreated patients with recurrent or progressive pediatric low-grade glioma.

The TriNetra™-Glio liquid biopsy has received breakthrough device designation from the FDA for the diagnosis of brain tumors.

The FDA has followed the recommendation of its Oncologic Drugs Advisory Committee and issued a complete response letter denying approval to 131I-omburtamab as a treatment for central nervous system and leptomeningeal metastases stemming from neuroblastoma.