
Chronic myeloid leukemia (CML) patients with higher copayments for the tyrosine kinase inhibitor imatinib were more likely to discontinue the drug or be non-adherent, according to a new study.

Your AI-Trained Oncology Knowledge Connection!


Chronic myeloid leukemia (CML) patients with higher copayments for the tyrosine kinase inhibitor imatinib were more likely to discontinue the drug or be non-adherent, according to a new study.
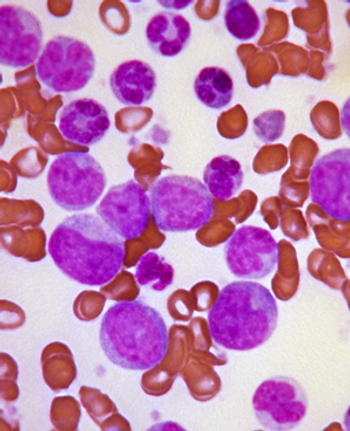
A deep molecular response to imatinib, achieved by most chronic myeloid leukemia patients who receive the drug, is predictive of better overall survival, according to a new study.
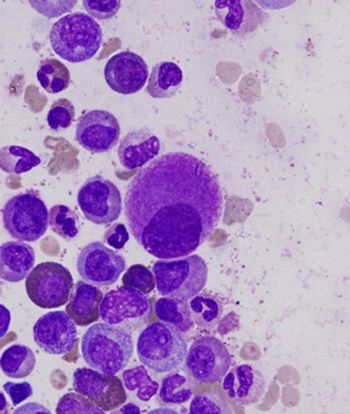
Modulation of the bone marrow microenvironment with parathyroid hormone may be a feasible way to dramatically reduce counts of leukemia stem cells in chronic myeloid leukemia patients, according to new research in mice.
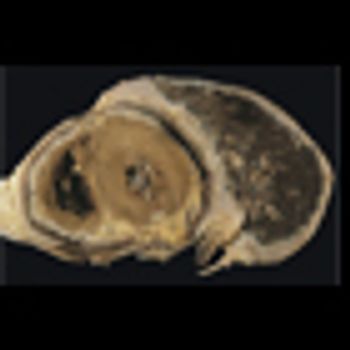
Mesotheliomas that arise after patients receive radiation therapy for lymphoma have unusual histologic features, and those patients tend to be younger and tend to survive longer than more common asbestos-related mesothelioma patients.
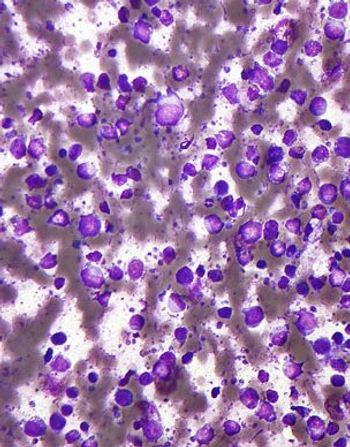
A new phase II trial found that a regimen containing rituximab, gemcitabine, cyclophosphamide, vincristine, and prednisolone is active and reasonably safe in patients with diffuse large B-cell lymphoma and coexisting cardiac disease.
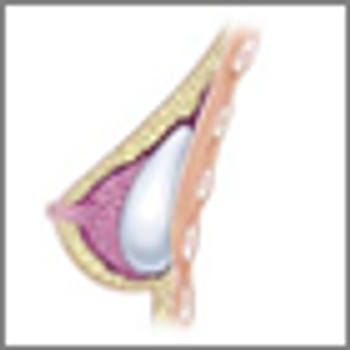
A study aimed at defining the natural history of breast implant-associated anaplastic large-cell lymphoma found that outcomes differ between those cases where the disease is confined within the fibrous capsule surrounding the implant and those where a mass is present in the breast.

Although the prospect is tempting, we do not believe there are sufficient grounds at this time to abandon bone marrow biopsy in patients with lymphoma. It still provides robust prognostic information, and in the majority of patients it remains an indispensable staging tool.

Clearly, eliminating a bone marrow biopsy in appropriate patients would be another step in the direction of minimizing the torture to which they are subjected.
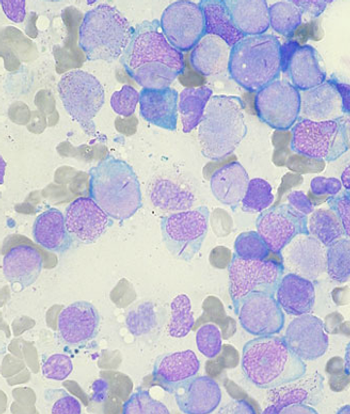
Treatment with gemtuzumab ozogamicin improved the event-free survival in children and adolescents with acute myeloid leukemia by reducing the risk of relapse among those able to achieve remission, according to trial results presented at the 2013 ASH meeting.
Ponatinib showed significant antileukemic activity in patients with CML and ALL, according to a phase II study that included a wide range of disease stages and mutation status; patients in the trial had a relatively high rate of adverse thrombotic events, an issue which has led to recent regulatory controversy surrounding the drug.

Following recent trial data showing an increased risk of dangerous blood clots with ponatinib, which led the FDA to request that the manufacturer stop marketing the drug, the FDA’s European counterpart has adjusted its recommendations for ponatinib but has not changed its “positive opinion” that led to the approval.

Women with airborne allergies to plants, grass, and trees may have a moderate increased risk of developing blood cancers, according to a new study.

The FDA has approved ibrutinib (Imbruvica) for the treatment of patients with mantle-cell lymphoma (MCL), an aggressive and rare blood cancer.
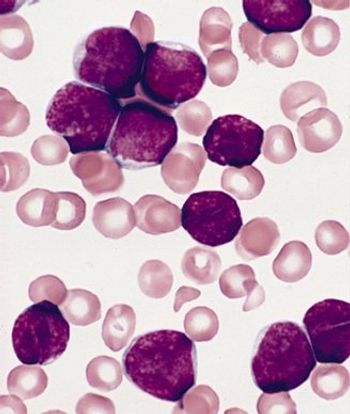
Adult survivors of pediatric acute lymphoblastic leukemia (ALL) suffer from persistent and significant neurocognitive deficits, according to a new study.
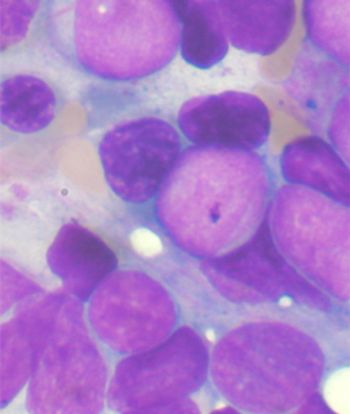
The sequential combination of gemtuzumab ozogamicin and standard chemotherapy offered no benefit in older patients with AML, and led to significant toxicities in those aged at least 70 years, according to results of a new study.

In the wake of reports that the drug ponatinib (Iclusig) can significantly increase risk of dangerous cardiovascular events, the FDA has asked the manufacturer to suspend sales and marketing of the drug in the United States.
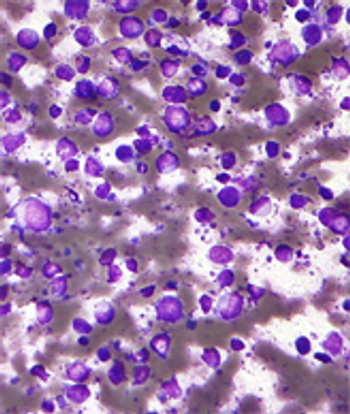
An 8-year study in patients with intermediate- to high-risk non-Hodgkin lymphoma shows that an autologous transplant following induction chemotherapy improves survival in high-risk patients.

The FDA issued a safety alert and reports it is investigating a high rate of thrombosis and other cardiovascular events in patients taking ponatinib (Iclusig) for CML or Philadelphia chromosome-positive ALL.
Patients with primary central nervous system lymphoma had high response rates and good long-term disease control with a chemotherapy regimen followed by consolidation reduced-dose whole-brain radiotherapy and cytarabine in a new phase II trial.

The role of transplant in MCL is in clinical evolution. Up-front high-dose therapy and autologous stem cell transplant remains an attractive option for those with chemosensitive disease regardless of the induction regimen chosen, whereas this approach in the relapsed or refractory setting has not yielded long-term disease-free intervals.

This review will cover innovative therapeutic approaches in relapsed or refractory MCL, many of which have the potential to alter treatment paradigms toward the development of strategies that do not involve conventional chemotherapy agents.

A mutation of the PAX5 gene has been found to play an important role in inherited cases of B-cell precursor acute lymphoblastic leukemia (B-ALL). The mutation was isolated in two unrelated families with high propensity for B-ALL.

Gilead Sciences has submitted an NDA to the FDA for its new drug idelalisib, for the use in patients with indolent non-Hodgkin lymphoma that is refractory to rituximab and chemotherapy.

Evidence continues to mount that discontinuing imatinib treatment for chronic myeloid leukemia (CML) in the chronic phase is safe. A new phase II Dutch and Belgian study showed only about two-thirds of patients relapsed after discontinuing treatment with imatinib and cytarabine, and all patients remained sensitive to imatinib after relapse.

MZL comprises three different entities that require integration of clinical and pathologic features to make a diagnosis. Treatment is chosen and initiated on the basis of presentation, symptoms, and underlying subtype.