Management of Midostaurin-CYP3A4 Drug-Drug Interactions in Patients With Acute Myeloid Leukemia
This article summarizes available data for the use of CYP3A4 inhibitors with midostaurin for acute myeloid leukemia.
Danielle Schlafer, PharmD, BCOP

TABLE 1: Management of Select Midostaurin Adverse Events[5]
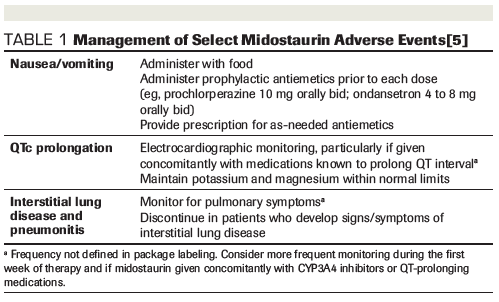
TABLE 2: Comparison of Select Antifungal Agents[14,21-24]
Background
Acute myeloid leukemia (AML) is the most common acute leukemia in adults, with an estimated 21,450 new cases diagnosed annually in the United States.[1] Approximately one third of patients with newly diagnosed AML harbor a mutation in the fms-like tyrosine kinase 3 (FLT3) gene, which encodes a receptor tyrosine kinase involved in proliferation and survival of hematopoietic progenitor cells.[2]Mutations in FLT3 are associated with poor outcomes with traditional chemotherapy. In particular, the internal tandem duplication FLT3 mutation is associated with decreased disease-free survival and shorter overall survival (OS) vs patients with wild-type FLT3.[3,4]
Midostaurin, a first-generation multitargeted tyrosine kinase inhibitor, was the first FLT3 inhibitor to be Food and Drug Administration (FDA) approved for use in AML in the United States.[5] It is indicated for use in the treatment of patients with newly diagnosed FLT3+ AML in combination with standard induction and consolidation chemotherapy. Its August 2017 approval ended a 17-year drought in new drug approvals for AML and was the first in a wave of targeted agents to be approved for the disease, including the second-generation inhibitor gilteritinib, approved in November 2018 for single-agent treatment of relapsed/refractory FLT3+ AML.[6]
The availability of these agents represents a paradigm shift in the treatment of FLT3-mutated AML, and clinicians should be aware of their unique toxicity profiles and prescribing considerations. Of particular interest is the management of drug-drug interactions mediated via cytochrome P450 (CYP) 3A4, as the use of interacting supportive care medications is common during induction and consolidation. This article summarizes available data for the use of CYP3A4 inhibitors with midostaurin.
Dosing and Adverse Events
FDA approval of midostaurin was based on the phase III RATIFY trial, which randomized 717 patients with newly diagnosed FLT3+ AML to receive midostaurin or placebo (50 mg twice daily on days 8 to 21) in combination with standard induction and consolidation chemotherapy followed by single-agent maintenance therapy (50 mg twice daily on days 1 to 28). The midostaurin arm demonstrated improved OS (median 74.7 months vs 25.6 months; P = .009) and event-free survival (EFS) (median 8.2 months vs 3.0 months; P = .002) vs placebo.[7]
Adverse events observed in the RATIFY study were comparable between the midostaurin and placebo treatment groups.7 The most frequent grade 3 or higher adverse events were hematologic toxicities, febrile neutropenia, infection, diarrhea, hypokalemia, pain, and increased alanine aminotransferase. Rates of grade ≥3 anemia (92.7% vs 87.8%, P = 0.03) and rash (14.1% vs 7.6%, P = .008) were higher with midostaurin versus placebo. While grade ≥3 nausea occurred more frequently in the placebo group (5.6% vs 9.6%, P =.05), the rates of all-grade nausea (83% vs 70%) and vomiting (61% vs 53%) were more common with midostaurin.[5,7] Toxicities occurring in more than 20% of patients in the midostaurin group included mucositis, febrile neutropenia, petechiae, headache, musculoskeletal pain, epistaxis, infection, and hyperglycemia.
Data from a phase Ib study of midostaurin in combination with chemotherapy in AML suggest that the drug’s tolerability is dose-dependent.[8] Dose schedules of 50 mg twice daily and 100 mg twice daily, both concomitantly with chemotherapy and sequentially after chemotherapy, were compared. Increased rates of grade 3/4 gastrointestinal adverse events were observed with the 100-mg schedule, including nausea/vomiting (24.1%) and diarrhea (13.8%), contributing to a 79% discontinuation rate. In contrast, there were no incidences of grade 3/4 nausea/vomiting in patients who received the 50-mg twice-daily schedule, and the discontinuation rate was lower at 45%.[8]
Additional safety concerns reported with midostaurin include QTc prolongation, which was higher in patients receiving midostaurin vs placebo, with elevations >480 ms and >500 ms occurring in 10.1% vs 5.7% and 6.2% vs 2.6% of patients, respectively. Pneumonitis and interstitial lung disease, including some fatal cases, have been reported in patients receiving midostaurin as a single agent or in combination with chemotherapy.[5] Monitoring recommendations are summarized in Table 1.
Pharmacokinetics and Drug-Drug Interactions
Midostaurin and its two active metabolites, CGP62221 and CGP52421, exhibit time-dependent pharmacokinetics with an initial increase in minimum concentrations during the first week of therapy followed by a decrease to steady-state levels.[5,9] When administered at 50 mg twice daily for 14 days sequentially after chemotherapy, trough concentrations of midostaurin and CGP62221 decrease to undetectable levels during off-treatment periods, while CGP52421 displays a long half-life and is maintained at detectable levels between dosing phases.[8] Midostaurin is primarily metabolized by CYP3A4, which catalyzes hydroxylation and demethylation to active metabolites.[10] Drug excretion is 95% via the hepatobiliary route, with 91% as metabolites and 4% excreted unchanged.[5]
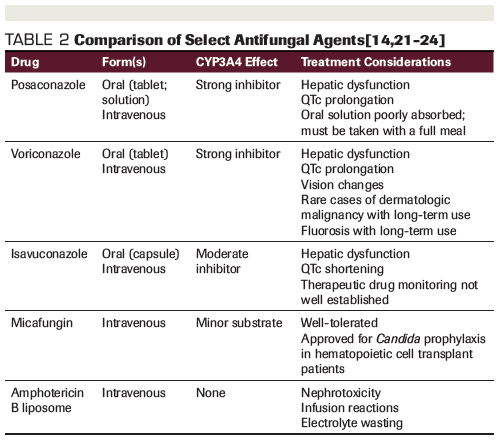
Coadministration of midostaurin with strong CYP3A4 inducers leads to reduced concentrations of midostaurin and its metabolites, with more than ten-fold decreased exposure.[9] The US package insert recommends avoiding coadministration of midostaurin with strong CYP3A4 inducers because of concern for reduced efficacy.[5] Concomitant administration of strong CYP3A4 inhibitors leads to increased exposure to midostaurin and its active metabolites. Inhibition of CYP3A4 by ketoconazole resulted in a 1.8-fold increase in midostaurin maximum concentration and delayed formation and excretion of its active metabolites.[9] Due to a theoretical concern for increased toxicity, drug labeling recommends the using alternative therapies that do not inhibit CYP3A4 or monitoring patients for increased risk of toxicity if coadministration with strong inhibitors cannot be avoided.[5] While the use of strong CYP3A4 inducers (eg, rifampin, phenytoin) is relatively uncommon, the use of strong CYP3A4 inhibitors (eg, posaconazole, voriconazole) for prophylaxis of fungal infections is a mainstay of AML supportive care.[3,11]
Use of concomitant CYP3A4 inhibitors as prophylaxis against fungal infection was permitted in the RATIFY study, without midostaurin dose adjustment. Fluconazole, posaconazole, and voriconazole were the most commonly prescribed agents. Use of concomitant strong CYP3A4 inhibitors occurred throughout induction, consolidation, and maintenance in 60.8%, 45.6%, and 10.8% of patients, respectively.[12] Midostaurin plasma levels were measured in a subset of 188 patients, demonstrating a 1.44-fold increase in midostaurin exposure in patients who received concomitant strong CYP3A4 inhibitors vs those who did not. The median dose intensity was similar (>94% of intended dose) regardless of concomitant strong CYP3A4 inhibitor therapy. Midostaurin-related adverse events were not significantly increased in patients receiving strong CYP3A4 inhibitors. However, an exposure-safety analysis showed a shorter time to first grade 3 or 4 adverse event in patients with higher midostaurin exposure, after accounting for presence of a CYP3A4 inhibitor (median 36 days vs 49 days). Higher dose intensity was associated with a benefit in EFS and OS, and presence of CYP3A4 inhibitors did not have a significant impact on efficacy outcomes.[12]
Considering these results, clinicians should be reassured that concomitant use of strong CYP3A4 inhibitors is not a contraindication to midostaurin therapy, but they should continue to be vigilant in monitoring for midostaurin-related toxicities. Aggressive prophylaxis and treatment of nausea and vomiting are warranted. Patients should be counseled on the use of scheduled prophylactic antiemetics and provided with an appropriate breakthrough antiemetic regimen.[5,13] Given the potential for QTc prolongation with several classes of antiemetics and select azole antifungals (Table 2), clinicians should strongly consider regular electrocardiographic monitoring and electrolyte replacement when initiating midostaurin and throughout therapy. Since QTc prolongation has been reported with the strong CYP3A4 inhibitors posaconazole and voriconazole, isavuconazole is a potentially attractive alternative given its moderate CYP3A4 inhibition and lack of QTc prolongation.[14] One case report described the successful concomitant administration of midostaurin and isavuconazole with no observed increase in toxicity.[15] It should be noted, however, that data are somewhat limited for prophylactic use of isavuconazole in immunocompromised patients, with some conflicting reports of breakthrough invasive fungal infections.[16-20]
Midostaurin is currently the only FDA-approved treatment for newly diagnosed FLT3+AML, demonstrating improved survival in a patient population with historically poor outcomes. Drug-drug interactions should be carefully reviewed at treatment initiation and throughout therapy with consideration of patient-specific risk factors and comorbidities. Alternative therapies should be considered when feasible. When no acceptable alternatives exist, midostaurin may be successfully coadministered with CYP3A4 inhibitors with cautious monitoring.
Financial Disclosure:Dr. Schlafer has no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
References:
1. SEER Cancer Stat Facts: Acute myeloid leukemia. Available at: https://seer.cancer.gov/statfacts/html/amyl.html. Accessed June 1, 2019.
2. Gilliland DG, Griffin JD. The roles of FLT3 in hematopoiesis and leukemia. Blood. 2002;100(5):1532-1542.
3. National Comprehensive Cancer Network. Acute myeloid leukemia (version 3.2019). Available at: https://www.nccn.org/professionals/physician_gls/pdf/aml.pdf. Accessed June 1, 2019.
4. Frohling S, Schlenk RF, Breitruck J, et al. Prognostic significance of activating FLT3 mutations in younger adults (16 to 60 years) with acute myeloid leukemia and normal cytogenetics: a study of the AML Study Group Ulm. Blood. 2002;100(13):4372-4380.
5. Rydapt (midostaurin) [prescribing information]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; June 2018.
6. Xospata (gilteritinib) [prescribing information]. Northbrook, IL: Astellas Pharma US, Inc; May 2019.
7. Stone RM, Mandrekar SJ, Sanford BL, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. 2017;377(5):454-464.
8. Stone RM, Fischer T, Paquette R, et al. Phase IB study of the FLT3 kinase inhibitor midostaurin with chemotherapy in younger newly diagnosed adult patients with acute myeloid leukemia. Leukemia. 2012;26(9):2061-2068.
9. Dutreix C, Munarini F, Lorenzo S, et al. Investigation into CYP3A4-mediated drug-drug interactions on midostaurin in healthy volunteers. Cancer Chemother Pharmacol. 2013;72(6):1223-1234.
10. RYDAPT (midostaurin) capsules pharmacology review. Silver Spring, MD: US Food and Drug Administration; 2017.
11. Cornely OA, Maertens J, Winston DJ, et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med. 2007;356(4):348-359.
12. Ouatas T, Duval V, Sinclair K, et al Concomitant use of midostaurin with strong CYP3A4 Inhibitors: An analysis from the Ratify trial. Blood. 2017;130(suppl 1):3814-3814.
13. National Comprehensive Cancer Network. Antiemesis (version 1.2019). Available at: https://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf. Accessed June 8, 2019.
14. Cresemba (isavuconazonium) [prescribing information]. Northbrook, IL: Astellas Pharma US Inc; April 2018.
15. Tollkuci E. Isavuconazole therapy in an FLT3 mutated acute myeloid leukemia patient receiving midostaurin: A case report. J Oncol Pharm Pract. 2019;25(4):987-989.
16. Bowen CD, Tallman GB, Hakki M, et al. Isavuconazole to prevent invasive fungal infection in immunocompromised adults: Initial experience at an academic medical centre. Mycoses. 2019. May 3. doi: 10.1111/myc.12924. [Epub ahead of print]
17. Cornely OA, Bohme A, Schmitt-Hoffmann A, et al. Safety and pharmacokinetics of isavuconazole as antifungal prophylaxis in acute myeloid leukemia patients with neutropenia: results of a phase 2, dose escalation study. Antimicrob Agents Chemo. 2015;59(4):2078-2085.
18. Fontana L, Perlin DS, Zhao Y, et al. Isavuconazole prophylaxis in patients with hematologic malignancies and hematopoietic-cell transplant recipients. Clin Iinfect Dis .2019 Apr 8. pii: ciz282. doi: 10.1093/cid/ciz282. [Epub ahead of print]
19. Fung M, Schwartz BS, Doernberg SB, et al. Breakthrough invasive fungal infections on isavuconazole prophylaxis and treatment: What is happening in the real-world setting? Clin Iinfect Dis. 2018;67(7):1142-1143.
20. Hassouna H, Athans V, Brizendine KD. Real-world use-Isavuconazole at a large academic medical center. Mycoses. 2019;62(6):534-541.
21. Noxafil (posaconazole) [prescribing information]. Whitehouse Station, NJ: Merck; March 2019.
22. Vfend (voriconazole) [prescribing information]. New York, NY: Pfizer; January 2019.
23. Mycamine (micafungin) [prescribing information]. Northbrook, IL: Astellas Pharma Inc; April 2018.
24. AmBisome (amphotericin B [liposomal]) [prescribing information]. San Dimas, CA: Gilead Sciences, Inc; July 2018.
