Ardaman Shergill, MD, and Aparna Raj Parikh, MD, explore ongoing clinical investigations evaluating the use of circulating tumor DNA in the treatment of early-stage colon cancer.

Your AI-Trained Oncology Knowledge Connection!


Ardaman Shergill, MD, and Aparna Raj Parikh, MD, explore ongoing clinical investigations evaluating the use of circulating tumor DNA in the treatment of early-stage colon cancer.
Phase 2/3 Study of Circulating Tumor DNA as a Predictive Biomarker in Adjuvant Chemotherapy in Patients With Stage IIA Colon Cancer (COBRA): a first-in-kind trial seeks to provide level 1 evidence in evaluating the role of ctDNA as a guide for clinicians in the decision to administer adjuvant chemotherapy to patients with stage IIA colon cancer (NRG GI-005) (NCT04068103).
Colon Adjuvant Chemotherapy Based on Evaluation of Residual Disease (CIRCULATE-US): a prospective phase 2/3 trial of MRD-based adjuvant therapy for patients with early-stage colon cancer with intensified and deintensified adjuvant therapy approaches using ctDNA status as a surrogate for MRD status (NRG-GI008) (NCT05174169).
Ibrahim Halil Sahin, MD, and colleagues, explore, the CIRCULATE-US (NRG-GI008; NCT05174169) investigating postoperative ctDNA dynamics in early-stage colon cancer for treatment selection.
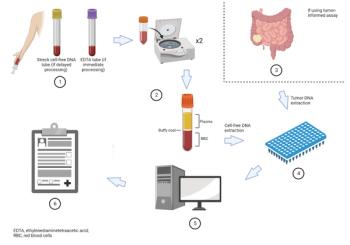
Ben Fangman, MD, and colleagues provide an overview of the use of circulating tumor DNA levels to detect minimal residual disease in colorectal cancer.

In an interview for Breast Cancer Awareness Month, Paolo Tarantino, MD, discusses the importance of multidisciplinary care for patients with breast cancer, the most exciting data presented this year, and prospects for future research.
Co-editor-in-Chief Howard S. Hochster, MD, highlights exciting clinical trials that may set future standards for adjuvant therapy selection in colon cancer.