Articles by Axel Grothey, MD

There is clear proof of principle for adjuvant therapy in patients at high risk for tumor recurrence, such as those with resected metastatic colorectal cancer. For that reason, this strategy has been largely adopted, especially using 5-FU– and oxaliplatin-based regimens, thereby mirroring the approach in resected stage III colon cancer.

Ahead of the ESMO World Congress on Gastrointestinal Cancer, we are discussing the use of maintenance therapy in metastatic colorectal cancer with Axel Grothey, MD.

Points made in commentaries on “Colorectal Cancer: How Emerging Molecular Understanding Affects Treatment Decisions” are addressed by authors Hubbard and Grothey.
In this review we discuss the current treatment options in metastatic colon cancer, with a special focus on biologic agents and how molecular understanding guides treatment decisions.

In this supplement to Oncology, guest editor Axel Grothey from the Mayo Clinic, explores adjuvant therapy considerations in stage II colon cancer from different angles.

it is obvious that some patients with stage II cancers have excellent outcomes and do not require additional therapy, whereas other patients have cancers that biologically behave more like stage III tumors.Over the past years, our understanding of stage II colon cancer has made significant advances leading to a refinement in the identification of the patient population and the treatment options considered appropriate as adjuvant therapy in this setting.

Davies/Goldberg Article Reviewed. The past decade has seen exciting developments in the field of colorectal cancer, particularly in the setting of advanced disease.

Over the past decade, new cytotoxic and biologic therapies beyond the old standard-of-care, biomodulated fluorouracil (5-FU), have become available for the treatment of metastatic colorectal cancer (mCRC). The introductions of irinotecan (Camptosar), oxaliplatin (Eloxatin), and bevacizumab (Avastin) have prolonged survival, but the optimal use of these new therapies remains to be determined. Issues remain regarding management of toxicities, treatment of elderly patients or those with poor performance status, and the duration of treatment with front-line therapy. This article reviews recent and ongoing studies of newer therapies in an effort to determine the best use of these drugs in the treatment of mCRC. Current data support the front-line use of bevacizumab added to either 5-FU/leucovorin alone or 5-FU/leucovorin in combination with oxaliplatin (FOLFOX/bevacizumab) or irinotecan (FOLFIRI/bevacizumab). If oxaliplatin is used in first-line therapy, oxaliplatin should be discontinued before the development of severe neurotoxicity and be reintroduced or replaced with irinotecan on disease progression. Definitive conclusions on the sequence and duration of front-line therapy and the most effective strategy to ameliorate toxicity await results of ongoing prospective clinical trials.

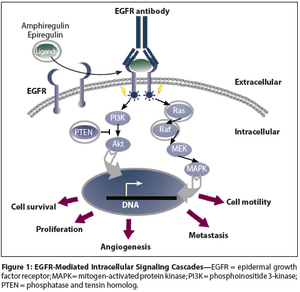
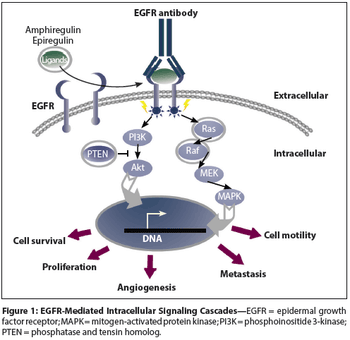
Traditional therapeutic concepts and treatment regimens for colorectal cancer are currently changing with the demonstration of the efficacy of biologic agents in this disease setting. The addition of the anti-vascular endothelial growth factor (VEGF) monoclonal antibody bevacizumab (Avastin) to conventional chemotherapy in the first- and second-line settings has shown a survival benefit; this outcome has helped to rapidly change the standard of care. Other targeted agents, such as anti-epidermal growth factor receptor (EGFR) antibodies, have shown proof of efficacy in colorectal cancer as well. The molecular targeted therapies are associated with toxicity profiles that are distinctly different from those seen with conventional chemotherapy. A notable difference is the absence of high risk for myelosuppression, diarrhea, or alopecia, which are common side effects of cytotoxic chemotherapy. This article will explore the toxicities associated with targeted therapies in detail in an attempt to provide assistance to the practicing oncologist in detecting and managing these side effects in their patients. In particular, the article will focus on the side effects associated with the three currently approved targeted drugs: the anti-VEGF monoclonal antibody bevacizumab and the anti-EGFR monoclonal antibodies cetuximab (Erbitux) and panitumumab (Vectibix).

Although the idea of utilizing antiangiogenic agents to hinder tumor growthis not new,[1] and the discovery of angiogenesis in tumors is evenolder,[2] this field of research is still in its infancy. Much has been learnedabout angiogenesis in tumor growth and development, yet the process is exceedinglycomplex and tightly regulated by a sophisticated interplay between pro- andantiangiogenic factors. Decades will pass before these regulatory mechanisms arewell elucidated and understood.

In the past 5 years, the treatment ofmetastatic colorectal cancer hasseen unparalleled advances. Medianoverall survivals reported inphase III trials have almost doubledand are poised to break the 2-yearbarrier very soon, perhaps as early asthis year. This has been made possiblethrough the introduction of a varietyof active agents into the treatmentof this disease.