Articles by John L. Marshall, MD

Panelists discuss how research on circulating tumor DNA (ctDNA) testing in colorectal cancer requires careful consideration of standardized collection protocols, analytical validation, clinical utility assessment, integration with existing biomarkers, and longitudinal monitoring to establish its role in personalized treatment decision-making.

Panelists discuss how circulating tumor DNA (ctDNA) testing serves as a complementary tool to traditional imaging in challenging colorectal cancer cases, offering molecular-level insights that can detect disease recurrence earlier, resolve ambiguous radiographic findings, and inform treatment decisions when conventional assessment methods yield inconclusive results.

Panelists discuss how adopting a patient-centric approach with comprehensive education about circulating tumor DNA (ctDNA) testing empowers patients with colorectal cancer (CRC) to make informed decisions about their surveillance strategy while advancing personalized care pathways for the future.

Panelists discuss how tumor-informed circulating tumor DNA (ctDNA) testing offers a personalized molecular surveillance strategy that can revolutionize colorectal cancer management through earlier detection of recurrence, real-time monitoring of treatment response, and potential for therapy de-escalation in patients with negative results.

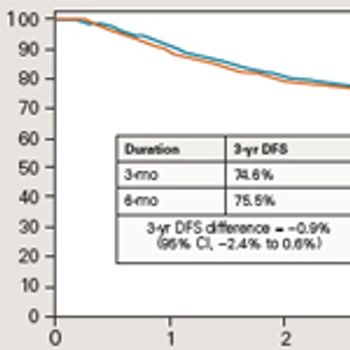
Panelists discuss how the efficacy data from SWOG 80702 demonstrated that shortened duration of adjuvant chemotherapy (3 months vs 6 months) yielded comparable survival outcomes while significantly reducing treatment-related toxicities, challenging previous standard-of-care approaches for patients with stage III colon cancer.

Panelists discuss how SWOG 80702, a large-scale randomized clinical trial, employed a robust factorial design with clearly defined efficacy end points, including disease-free and overall survival, while enrolling a diverse patient population with stage III colon cancer to evaluate optimal adjuvant therapy duration and the impact of celecoxib.

Panelists discuss how minimal residual disease (MRD) status, as detected through circulating tumor DNA analysis, can guide personalized treatment decisions by identifying patients who may benefit from intensification of adjuvant therapy and those who could safely undergo de-escalation of treatment regimens.

Panelists discuss how circulating tumor DNA (ctDNA) clearance rates during and after adjuvant chemotherapy (ACT) in the BESPOKE trial provide critical insights into treatment efficacy and may serve as an early predictor of long-term patient outcomes.

Panelists discuss how BESPOKE trial results demonstrate significantly improved disease-free survival rates in patients with negative circulating tumor DNA (ctDNA) status compared with those with detectable ctDNA, underscoring its potential as a powerful prognostic biomarker.

Panelists discuss how the BESPOKE trial enrolled a diverse cohort of patients with varying demographic profiles, disease stages, comorbidities, and treatment histories to ensure comprehensive representation for personalized medicine approaches.

Panelists discuss how the BESPOKE study’s innovative design, carefully selected efficacy end points, and rigorous methodological approach aim to evaluate personalized therapeutic interventions with unprecedented precision.

Panelists discuss how circulating tumor DNA (ctDNA) serves as a promising biomarker for early detection, treatment monitoring, and recurrence surveillance in patients with colorectal cancer (CRC).

Data from the SPOTLIGHT and GLOW trials reveal that zolbetuximab increased survival in patients with CLDN18.2-positive gastric or GEJ adenocarcinoma.

The incorporation of zolbetuximab in addition to chemotherapy has shown benefit in patients with Claudin 18.2–positive gastric cancers in clinical trials.

The expert from the Otto J. Ruesch Center for the Cure of Gastrointestinal Cancers discusses the implications of precision medicine for cancer care, and the need to make that care more accessible for the global community at large.
A previously healthy 36-year-old man initially presented to his primary care physician with occasional bloody stools and dull right upper quadrant pain. Blood was sometimes mixed into his stools but was more often seen on the toilet paper after wiping.

In this review, we address specific issues pertaining to AYA patients with colorectal cancer, including evaluation for hereditary colorectal cancer syndromes, clinicopathologic and biologic features unique to AYA patients with colorectal cancer, treatment outcomes, and survivorship.

Molecular Testing to Optimize and Personalize Decision Making in the Management of Colorectal Cancer
ByMarwan Al-Hajeili, MD,Anthony F. Shields, MD, PhD,Jimmy J. Hwang, MD,Raymond C. Wadlow, MD,John L. Marshall, MD Recent improvements in our understanding of the biology of colorectal cancer have led to the identification of several important prognostic and predictive markers of disease-associated risk and treatment response for the individual patient.

Here we discuss the evolution of standard therapy for rectal cancer patients and the use of preoperative CRT for the treatment of locally advanced disease. Treatment schemes that have attempted to broaden the horizons of standard therapy include the use of induction chemotherapy and “watch-and-wait” approaches.

Here, we review the studies that have explored different treatment regimens, therapeutic sequencing, and biologic inclusions for the treatment of these patients, with neoadjuvant intent. We also describe how we have established our own treatment paradigm for the management of potentially curable metastatic colorectal cancer.

In all other settings, giving chemotherapy just to make us feel better emotionally in exchange for potential harm to the patient is not the right strategy. We need to find better treatments for this new clinical indication.

We have yet to establish a standard practice for maintenance therapy in metastatic colorectal cancer. Of course, ideally we could find a biomarker of benefit for patients who should be managed this way, but thus far we have had no such luck.

In this Stump the Professor video, two fellows test Dr. Marshall's diagnostic skills with a unique case study involving an 80-year-old patient who presents with abdominal pain, bilateral edema, weight loss, and dark, tarry stools.

During the past decade, targeted therapeutics have changed the landscape of cancer therapy with bold brush strokes, but the resulting images are still unclear. As we enter into the second decade of targeted therapy, our increased understanding of tumor biology together with cancer genomic sequencing tools will clearly show the way forward. It is imperative that we use these fine brushes, not only to improve precision, but in the end to realize the art of medicine.

In my practice as an oncologist specializing in gastrointestinal tract cancers, a recent week was fairly typical. I saw 50 patients, ranging in age from 32 to 87, equally divided between men and women. Though a couple of them have inherited a gene that may have caused their GI cancers, I have no explanation for why most developed their disease. It is as if they were simply struck by lightning.

In the treatment of colon cancer today, the decision-making involved in the treatment of stage II disease is probably the most challenging aspect. The major question is whether or not these patients should receive postoperative adjuvant chemotherapy. Approximately 75% of stage II colon cancer is cured by surgery alone. For the remaining 25% of cases, there is great debate over whether adjuvant chemotherapy is sufficiently effective in enough patients to warrant the exposure to potentially toxic treatments. In the important QUASAR clinical trial, stage II patients were randomized to either fluorouracil (5-FU)-based therapy or observation. The results demonstrated an approximate 3% improvement in outcome for the 5-FU–treated patients. This leads to the assumption that treating all stage II patients with adjuvant chemotherapy is gross overtreatment, when essentially 97% of these patients will not benefit. Clearly the only way to approach this decision is through risk determination. In this article, I will describe the current state of defining high- and low-risk disease, which is mainly through histopathologic characteristics, as well as discuss emerging approaches such as molecular markers and genomic profiling

Preliminary results from two trials presented at the 44th Annual Meeting of ASCO in Chicago have consolidated the role of K-ras as a biomarker of nonresponse to cetuximab and panitumumab in metastatic colorectal cancer (mCRC). The phase III CRYSTAL and OPUS trials presented unplanned subgroup analyses of the correlation of K-ras status with response to therapy with first-line FOLFIRI or FOLFOX, respectively, with or without cetuximab in patients with mCRC. Both studies demonstrated a clear benefit with the addition of cetuximab in K-ras WT patients.

I will never forget the moment when I first found out about my wife's breast cancer. She had noticed an abnormal thickening in her breast and even though we were spending most of our attention focused on an adenoma on the opposite side, the surgeon felt that a biopsy would be prudent. It was a Monday morning, and I was at my desk. Because of my position within our institution, I happened to be on the distribution list of my wife's pathology report. However, before I got to that point in the mail, my close colleague came to my office to notify me of her diagnosis of invasive breast cancer.

The majority of patients who undergo resection for gastric cancer experience relapse and ultimately die of their disease. Therefore, considerable attention has been paid to neoadjuvant and adjuvant strategies to improve surgical outcomes. Two different approaches have been tested in major clinical trials conducted in the past several years: Postoperative chemoradiotherapy was assessed in a US Southwest Oncology Group/Intergroup study (SWOG 9008/INT 0116), and perioperative chemotherapy was studied in a UK Medical Research Council (MRC) randomized trial (the MRC Adjuvant Gastric Infusional Chemotherapy [MAGIC] trial). These trials demonstrated statistically significant survival benefits in patients with resectable gastric cancer. This review will consider these trials and their implications for clinical practice.

Over the past decade, new cytotoxic and biologic therapies beyond the old standard-of-care, biomodulated fluorouracil (5-FU), have become available for the treatment of metastatic colorectal cancer (mCRC). The introductions of irinotecan (Camptosar), oxaliplatin (Eloxatin), and bevacizumab (Avastin) have prolonged survival, but the optimal use of these new therapies remains to be determined. Issues remain regarding management of toxicities, treatment of elderly patients or those with poor performance status, and the duration of treatment with front-line therapy. This article reviews recent and ongoing studies of newer therapies in an effort to determine the best use of these drugs in the treatment of mCRC. Current data support the front-line use of bevacizumab added to either 5-FU/leucovorin alone or 5-FU/leucovorin in combination with oxaliplatin (FOLFOX/bevacizumab) or irinotecan (FOLFIRI/bevacizumab). If oxaliplatin is used in first-line therapy, oxaliplatin should be discontinued before the development of severe neurotoxicity and be reintroduced or replaced with irinotecan on disease progression. Definitive conclusions on the sequence and duration of front-line therapy and the most effective strategy to ameliorate toxicity await results of ongoing prospective clinical trials.










