Vaccines
Latest News
Latest Videos

Podcasts
CME Content
More News
Fareed Khawaja, MBBS, and colleagues provide a comprehensive overview of COVID-19 vaccine efficacy and safety among patients with cancer in the United States.

Those immunized between the ages of 12 and 13 in England experienced a significant estimated reduction in cervical cancer and grade 3 cervical intraepithelial neoplasia incidence rates compared with unvaccinated women.
UV1 has received fast track designation from the FDA for the use in unresectable or metastatic melanoma.
Seventeen years of data indicate that HPV-related cervical cancer incidence is declining, but other tumors related to the virus persist.
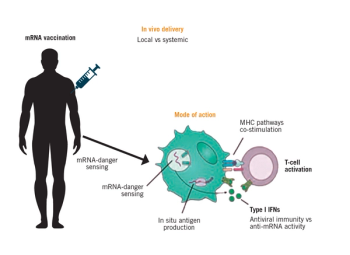
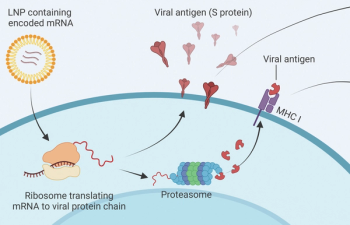
A review of mRNA vaccine technology by ONCOLOGY® editorial board member Mehmet Sitki Copur, MD, FACP, explains the basis for progress in this field and reviews a number of new trials using this technology.

In his "Letter to the Readers", co-editor-in-chief of the journal ONCOLOGY Howard S. Hochster, MD, reviews the development of mRNA technology, especially as it applies to vaccines against COVID-19.
In a sample of patients with comorbid conditions that included patients with cancer, nearly 20% of patients reported having reservations about receiving the COVID-19 vaccine.
The first single shot COVID-19 vaccine, and the third overall, to receive Emergency Use Authorization will start shipping to all parts of the United States immediately following a meeting of the FDA’s Vaccines and Related Biological Products Advisory Committee.