Lung Cancer
Latest News
Video Series

Latest Videos
Shorts
Podcasts
CME Content
More News

Data from the DeLLphi-304 trial support the full approval of tarlatamab in this extensive-stage small cell lung cancer population.

One patient with metastatic bladder cancer experienced an ongoing metabolic complete response following treatment with aldesleukin/imneskibart.
Data from a phase 1a/1b trial show that no patients discontinued STK-012 due to treatment-related adverse effects.

Dato-DXd is being assessed in numerous trials across the breast, lung, and bladder cancer spaces.

ILKN421H plus pembrolizumab previously showed antitumor activity among patients with frontline non–small cell lung cancer in a phase 1 trial.

According to Eyub Akdemir, MD, reducing EDIC may be feasible without compromising target coverage to reduce anticipated lymphopenia rates.

Data from the phase 2 TUXEDO-3 trial support patritumab deruxtecan as a novel treatment option across different cancer populations with brain metastases.

Data from the REZILIENT2 trial show meaningful intracranial activity in patients with NSCLC harboring EGFR exon 20 insertions or other uncommon mutations.

Although survival outcomes were numerically improved with ROS1-targeted therapies, an unmet need remains for patients with ROS1-mutated advanced NSCLC.

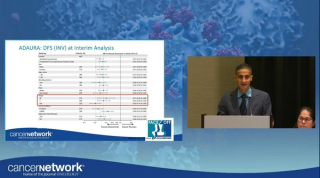
Alectinib exhibited a CNS DFS improvement, with a 63% reduction in the risk of this event, and 4-year CNS DFS rate was 90.4% vs 76.1% with chemotherapy.

Retrospective cohort findings may inform tailored treatment approaches for frontline metastatic BRAF V600E–mutated non–small cell lung cancer.

Data from the KEYNOTE-671 trial support the use of pembrolizumab among patients with non–small cell lung cancer in the perioperative setting.

Data from the phase 3 HARMONi-6 study may support ivonescimab plus chemotherapy as a new standard of care in advanced squamous non–small cell lung cancer.

Slow accrual led to the early stopping of the phase 3 DREAM3R trial assessing durvalumab/chemotherapy in advanced pleural mesothelioma.

OptiTROP-Lung04 data show sacituzumab tirumotecan cut the risk of progression or death by 51% in patients with nonsquamous EGFR-mutated NSCLC resistant to EGFR TKIs.

Osimertinib plus chemotherapy significantly improved overall survival in EGFR-mutated NSCLC, outperforming monotherapy across various prognostic factors.

Osimertinib and local consolidative therapy was a safe and effective strategy to extend disease control in patients with advanced EGFR-mutant NSCLC.

Alectinib shows promising long-term survival benefits over crizotinib for advanced ALK-positive NSCLC, highlighting significant clinical advancements.

Sevabertinib monotherapy was deemed tolerable across various cohorts of patients who were pretreated and treatment-naïve with HER2-mutant advanced NSCLC.

Results from the Beamion LUNG-1 study showed that first-line zongertinib yielded continued benefit for patients with HER2-mutated NSCLC.
The safety profile of durvalumab after radiotherapy was consistent with durvalumab after chemoradiotherapy among those with unresectable stage III NSCLC.

Trials slated for presentation at the 2025 ESMO Congress may reveal practice-changing data across different breast and lung cancer populations.

Developers plan to discuss a regulatory path to conditional marketing authorization for OST-HER2 in the UK, US, and EU in resected metastatic osteosarcoma.

Treatment-related AEs with sunvozertinib were consistent with EGFR tyrosine kinase inhibitors in patients with NSCLC with EGFR exon 20 insertion mutations.

Findings from the 2025 World Conference on Lung Cancer reflected key updates in the management of NSCLC, SCLC, and other lung cancer types.