
Safety data from ASCENT-03 support sacituzumab govitecan as an effective therapy with manageable toxicities in advanced triple-negative breast cancer.

Your AI-Trained Oncology Knowledge Connection!


Safety data from ASCENT-03 support sacituzumab govitecan as an effective therapy with manageable toxicities in advanced triple-negative breast cancer.

“VIKTORIA-1 is the first study to demonstrate a statistically significant and clinically meaningful improvement in PFS with PAM inhibition in patients with PIK3CA wild-type disease, all of whom received prior CDK4/6 inhibition,” said Barbara Pistilli, MD.

Zanubrutinib led to a 72% reduction in the risk of disease progression or death vs bendamustine/rituximab in this CLL/SLL population.

Results from the MagnetisMM-30 trial showed early ORR data with elranatamab/iberdomide in R/R multiple myeloma.

Datopotamab deruxtecan significantly enhanced survival rates in first-line treatment for metastatic triple-negative breast cancer.

Data from the phase 3 HARMONi-6 study may support ivonescimab plus chemotherapy as a new standard of care in advanced squamous non–small cell lung cancer.
Data from IDeate-Lung01 reveal a disease control rate exceeding 90% with infinatamab deruxtecan.

Enfortumab vedotin plus pembrolizumab enhanced survival rates for patients with muscle-invasive bladder cancer not eligible for cisplatin chemotherapy.
Updated results from SunRISe-4 support further investigation of the gemcitabine intravesical system plus cetrelimab in MIBC.

Osimertinib and local consolidative therapy was a safe and effective strategy to extend disease control in patients with advanced EGFR-mutant NSCLC.

AE-related discontinuations of osimertinib were low, and no new treatment-related deaths were reported with the combination in EGFR-mutant NSCLC.

Phase 1b data show antitumor activity with the givastomig combination across a wide range of CLDN18.2 expression.

Data from the TALENTACE trial support TACE plus atezolizumab/bevacizumab as an effective option in those with unresectable hepatocellular carcinoma.
Subgroup data from RATIONALE-306 support the frontline use of tislelizumab plus chemotherapy in locally advanced esophageal squamous cell carcinoma.
Subgroup data from BREAKWATER support cetuximab/encorafenib plus mFOLFOX6 as a new standard of care in BRAF V600E-mutated metastatic colorectal cancer.

Similar response rates and safety profiles were observed with subcutaenous and intravenous isatuximab in the phase 3 IRAKLIA study.
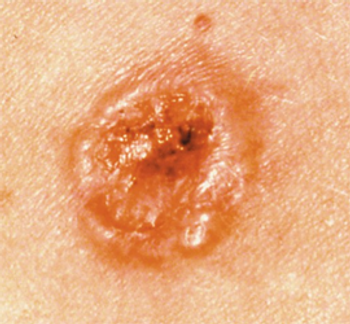
Compared with nivolumab alone, nivolumab/relatlimab did not improve RFS in patients with resected stage III to IV melanoma.

Results from CheckMate-816 could be practice-changing after an OS improvement was noted with NAT nivolumab plus chemotherapy in resectable NSCLC.

Data from KEYNOTE-A18 support pembrolizumab plus concurrent chemoradiotherapy as a standard of care in this cervical cancer population.

Subgroup data from CARTITUDE-4 suggest that cilta-cel may overcome the poor prognosis associated with some high-risk features in multiple myeloma.

A post hoc analysis of NAPOLI-3 reveals clinical characteristics and treatment strategies associated with long-term survival in patients with metastatic PDAC treated with NALIRIFOX.

Treatment with KITE-363 yields no dose-limiting toxicities in a first-in-human phase 1 study.

PFS was improved with first-line sacituzumab govitecan plus pembrolizumab for patients with PD-L1–positive metastatic TNBC.

The phase 3 IMforte trial evaluating lurbinectedin plus atezolizumab is the first to show PFS and OS improvement with first-line maintenance for ES-SCLC.
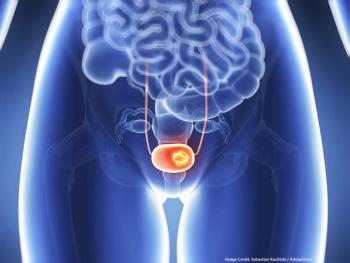
Results from the phase 3 ENVISION trial suggest UGN-102 could be a non-surgical alternative to TURBT for recurrent low-grade intermediate-risk NMIBC.

Mirvetuximab soravtansine additionally showcased PFS, ORR, and DOR benefits over chemotherapy in FRα-positive platinum-resistant ovarian cancer.

Idecabtagene vicleucel led to high rates of MRD negativity in multiple myeloma who responded did not have complete responses to first-line therapy.

Data from the EV-302 trial support enfortumab vedotin plus pembrolizumab as a new standard of care in frontline urothelial cancer.

Updated CARTITUDE-4 trial data show cilta-cel induces deep MRD negativity in patients with lenalidomide-refractory multiple myeloma.

Data from CheckMate-274 support adjuvant nivolumab as a standard of care in high-risk muscle-invasive urothelial carcinoma.
Published: April 14th 2021 | Updated:
Published: September 26th 2020 | Updated:

Published: February 11th 2021 | Updated:
Published: January 4th 2022 | Updated:
Published: June 7th 2021 | Updated:
Published: September 20th 2020 | Updated: