Combining pembrolizumab with cabozantinib produces encouraging efficacy in platinum-ineligible patients with advanced urothelial carcinoma, says Rohit K. Jain, MD, MPH.

Your AI-Trained Oncology Knowledge Connection!


Combining pembrolizumab with cabozantinib produces encouraging efficacy in platinum-ineligible patients with advanced urothelial carcinoma, says Rohit K. Jain, MD, MPH.
Investigators observe a benefit with durvalumab plus neoadjuvant chemotherapy among subgroups of patients regardless of microsatellite instability status in the phase 3 MATTERHORN trial.
Treatment with anito-cel produces deep, enduring responses in high-risk relapsed/refractory multiple myeloma, says Matthew Frigault, MD.
Phase 2 results showed that the investigational monoclonal antibody axatilimab elicited encouraging clinical activity and tolerability across dose levels in patients with recurrent or refractory chronic graft-vs-host disease.
Data from the phase 1/2 BRUIN study support continuous BTK pathway inhibition as an important sequencing approach in chronic lymphocytic leukemia or small lymphocytic lymphoma, says Jennifer A. Woyach, MD.
Treatment with atezolizumab among patients with HER2-positive breast cancer in the phase 3 APTneo Michelangelo trial produces no major tolerability issues, says Luca Gianni, MD.
The pembrolizumab-based regimen in the phase 3 KEYNOTE-756 trial produces a larger pathologic complete response benefit in patients with node-positive disease and higher PD-L1 expression, according to Joyce O’Shaughnessy, MD.
Patients with RET fusion–positive non–small cell lung cancer experienced improved progression-free survival with first-line selpercatinib.
Results from the phase 2 PERLA trial showed an improved objective response and overall survival in treatment with dostarlimab plus chemotherapy in patients with treatment-naïve, nonsquamous non–small cell lung cancer.

Findings from the phase 3 AEGEAN trial suggest that patients with EGFR-mutated non–small cell lung cancer experience similar outcomes whether receiving durvalumab or placebo.
Iruplinalib appears tolerable with no new safety signals among patients with locally advanced and metastatic ALK-positive non–small cell lung cancer in the phase 3 INSPIRE trial.
Benmelstobart plus anlotinib and chemotherapy produce survival benefits in extensive-stage small cell lung cancer across all patient subgroups in the phase 3 ETER701 trial.
A major molecular response at 3 months appears to increase the probability of overall survival in patients treated with ponatinib for chronic-phase chronic myeloid leukemia in the phase 2 PACE trial.
The phase 2 ORCHID trial met the respecified end point of Simon stage 1 design for patients with BAP1-mutated renal cell carcinoma.
Sotorasib maintains a progression-free survival benefit compared with docetaxel in the treatment of patients with KRAS G12C-mutated non–small cell lung cancer in the phase 3 CodeBreaK 200 trial.

Pembrolizumab plus axitinib also improves the overall response rate vs sunitinib alone among patients with clear cell renal cell carcinoma in the phase 3 KEYNOTE-426 trial.
Investigators report no deaths related to treatment with ABBV-011 among patients with small cell lung cancer in a first-in-human phase 1 trial.
![“We think that we can successfully de-escalate treatment of rectal cancer and achieve the same high cure rates [and] keep patients disease free, with less long-term toxicity and effects,” lead author Deb Schrag, MD, FASCO, MPH, said.](https://cdn.sanity.io/images/0vv8moc6/cancernetwork/852c505e728c0e9c7b27981b616ac51a65af1503-1200x800.jpg?w=350&fit=crop&auto=format)
“We think that we can successfully de-escalate treatment of rectal cancer and achieve the same high cure rates [and] keep patients disease free, with less long-term toxicity and effects,” lead author Deb Schrag, MD, FASCO, MPH, said.
Patients with DLL3-positive small cell lung cancer and neuroendocrine carcinoma experience early efficacy following treatment with BI 764532.
A real-world population of patients with relapsed/refractory B-cell acute lymphoblastic leukemia are reported to have had a high rate of complete remissions following treatment with brexucabtagene autoleucel.

A positive R0 resection rate helped to confirm the noninferiority of minimally invasive distal pancreatectomy compared with open distal pancreatectomy in those with resectable pancreatic cancer.
Durvalumab plus tremelimumab and hypofractionated radiotherapy yields observable clinical benefits in patients with gynecologic cancers, according to an expert from The University of Texas MD Anderson Cancer Center.
Patients with locally advanced or metastatic renal cell carcinoma with a clear-cell component who receive first-line cabozatinib after checkpoint inhibitor combination therapy appear to have early responses.
Patients with newly diagnosed, advanced biliary tract cancers do not have a statistically significant survival benefit following treatment with nab-paclitaxel plus gemcitabine and cisplatin.

Patients with previously untreated advanced gastric cancer, gastroesophageal junction cancer, and esophageal adenocarcinoma continue to benefit from treatment with nivolumab and chemotherapy.
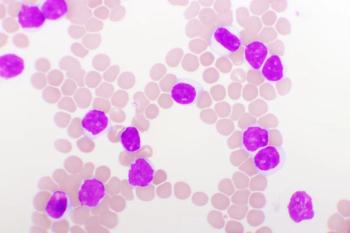
Ziftomenib monotherapy showed pronounced antileukemic activity and a tolerable toxicity profile in pretreated patients with relapsed/refractory acute myeloid leukemia.
The phase 3 ASAP trial found the use of watchful waiting followed by sequential conditioning prior to allogeneic hematopoietic cell transplantation found a similar survival benefit in patients with relapsed/refractory acute myeloid leukemia.
Final data from the phase 2 ATLEP trial showed high response rates with a combination of lenvatinib and pembrolizumab in patients with anaplastic and poorly differentiated thyroid cancer.
Findings from the phase 3 ADURA trial indicated that patients with EGFR-mutated, stage II to IIIA non–small cell lung cancer (NSCLC) experienced a promising reduction in risk of disease recurrence or death following treatment with adjuvant osimertinib.
Patients with with metastatic castration-resistant prostate cancer continued to derive notable benefit from treatment with first-line olaparib plus abiraterone acetate and prednisone or prednisolone compared with abiraterone monotherapy.