
Molecular profiling is being explored in pancreatic adenocarcinoma as a tool to assist with early detection, prognosis, and patient selection in targeted therapy clinical trials.

Your AI-Trained Oncology Knowledge Connection!


Molecular profiling is being explored in pancreatic adenocarcinoma as a tool to assist with early detection, prognosis, and patient selection in targeted therapy clinical trials.
This study published in the journal ONCOLOGY® characterizes molecular profiling practice patterns in individuals with advanced PDAC who were referred to a tertiary clinical trials drug development unit.
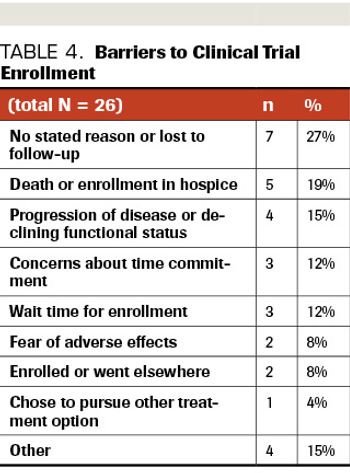
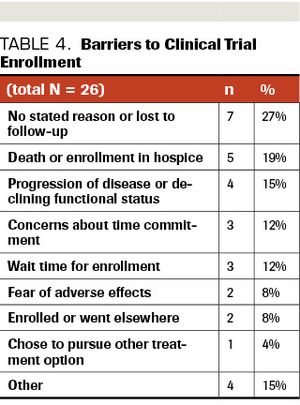
ABSTRACT BACKGROUND: Early-phase clinical trials are critical to the advancement of cancer care, especially in patients with pancreatic ductal adenocarcinoma, given its aggressive nature and limited available therapeutic options. METHODS: A retrospective chart review of all patients with refractory or metastatic pancreatic ductal adenocarcinoma, referred to the Sarah Cannon Research Institute at HealthONE between 2014 and 2019, were reviewed. Patients who completed genomic profiling and qualified for a phase 1 trial (primarily 1a but some 1b) were identified to assess barriers to trial enrollment. RESULTS: Of 74 identified patients, 54 patients (73%) qualified for at least 1 clinical trial based on eligibility criteria and alterations detected via molecular profiling. Up to 40 industry-sponsored clinical trials were available during this time for consideration. Of the 54, 28 patients (52%) enrolled in a clinical trial, while 26 (48%) did not enroll. The most frequently cited barriers to enrollment were concerns regarding time commitment (12%), prolonged wait time for enrollment (12%), and fear of adverse events (8%). Seven of the 26 patients (27%) were lost to follow-up or had no stated reason for declining enrollment; others did not go on trial due to death/transition to hospice (n=5; 19%) or progression of disease/declining functional status (n=4; 15%). There were few statistically significant differences between patients who chose to go on trial and those who declined. CONCLUSIONS: An understanding of why eligible patients elect not to participate in early-phase clinical trials provides insight into the patient experience and can help identify misperceptions and areas for improvement in education and the clinical trial enrollment process.

ABSTRACT: Disease progression or recurrence after a period of remission can be a challenging event for individuals seeking cancer treatment. Those referred for possible phase 1 trial enrollment are often motivated to participate in these studies with hope for a cure despite approximately 5% response rates in this setting. Addressing such commonly held misunderstandings during the initial evaluation for phase 1 trial eligibility could provide a valuable opportunity to improve physician communication by identifying signs of distress or psychiatric conditions, addressing underlying psychological biases, and encouraging adaptive coping strategies.
Published: October 14th 2020 | Updated:

Published: September 22nd 2020 | Updated:
Published: May 12th 2021 | Updated:
Published: December 23rd 2021 | Updated: