FDA Clears Non-Invasive AI Device for Skin Cancer Detection
The DermaSensor device demonstrates a high rate of sensitivity in the detection of more than 200 types of skin cancers in a clinical study.
The device’s mechanism is believed to assist practices reduce reliance on clinical training and subjective judgment that are otherwise necessary for assessing suspicious moles or lesions with the naked eye or standard magnified visual examination.
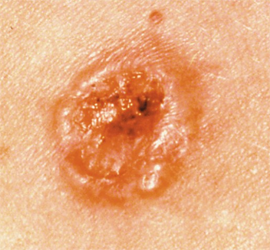
The FDA has granted clearance to DermaSensor’s artificial intelligence (AI)–powered tool for evaluating skin cancer, including melanoma, basal cell carcinoma, and squamous cell carcinoma, according to a press release from DermaSensor Inc.1
Developers designed the spectroscopy technology to help physicians non-invasively assess the cellular and subcellular features of a lesion suspected to confer skin cancer. By acting on an FDA-cleared algorithm, the AI-based tool can generate an immediate, objective result for a diagnosis. The device’s mechanism is believed to assist practices reduce reliance on clinical training and subjective judgment that are otherwise necessary for assessing suspicious moles or lesions with the naked eye or standard magnified visual examination.
“We are entering the golden age of predictive and generative [AI] in health care, and these capabilities are being paired with novel types of technology, like spectroscopy and genetic sequencing, to optimize disease detection and care,” Cody Simmons, co-founder and chief executive officer at DermaSensor, said in the press release.1 “Equipping primary care physicians [PCPs], the most abundant clinicians in the country, to better evaluate the most common cancer in the country has been a major, long-standing unmet need in medicine. While dozens of companies have attempted to address this problem in recent decades, we are honored to be the first device cleared by the FDA that provides PCPs with an automated tool for evaluation of suspicious lesions.”
The AI-based tool’s ability to detect skin cancer was evaluated in a study including over 1000 patients at 22 treatment centers. In the study, the device demonstrated a 96% sensitivity when detecting 224 skin cancers.2 Additionally, investigators highlighted that a negative reading conferred a 97% possibility of a benign skin cancer.
According to findings presented at the 2023 American Academy of Dermatology Innovation Academy, 72% of patients were classified as having Fitzpatrick skin type (FST) I to III, and 28% had FST IV to VI skin cancer.3 Additionally, 48 skin cancers were detected among those in the FST IV-VI group, and investigators noted minimal variation in the tool’s specificity, sensitivity, and Area under the Receiver Operating Characteristic Curve (AUROC) between the FST subgroups. The device demonstrated a sensitivity of 96% among patients in the FST I to III group and 92% among those with FST IV to VI skin cancer; the AUROC among patients in each respective group was 0.779 and 0.764.
“The findings of this sub-analysis are highly encouraging as they suggest that DermaSensor’s elastic scattering spectroscopy [ESS] device performance is not significantly impacted by underlying melanin content and can be a valuable tool in detecting skin cancer across different skin types, including patients with skin of color,” David J. Leffell, MDCM, professor of Dermatology, Plastic Surgery, and Otolaryngology and chief of the Dermatologic Surgery Program at the Yale School of Medicine as well as a member of the Scientific Avisory Board at DermaSensor, said in a press release on these findings.3
Findings from a companion clinical utility study highlighted that the rate of missed skin cancers was reduced from 18% to 9% with DermaSensor’s AI-based tool.4 Investigators concluded that the device helped physicians become more accurate and confident when evaluating cancerous lesions in the skin.
References
- FDA clearance granted for first AI-powered medical device to detect all three common skin cancers (melanoma, basal cell carcinoma and squamous cell carcinoma). News release. DermaSensor Inc. January 17, 2024. Accessed January 18, 2024. http://tinyurl.com/34jxj9xu
- Merry SP, Chatha K, Croghan I, Nguyen VL, McCormick B, Leffel D. Clinical performance of novel elastic scattering spectroscopy (ESS) in detection of skin cancer: A blinded, prospective, multi-center clinical trial. J Clin Aesthet Dermatol. 2023;16(suppl 4):s16.
- DermaSensor study results for skin cancer detection device performance across skin tones of over 1,000 patients. News release. DermaSensor Inc. August 10, 2023. Accessed January 18, 2024. http://tinyurl.com/5t3hfd6w
- Seiverling EV, Agresta T, Cyr P, et al. Clinical utility of an elastic scattering spectroscopy device in assisting primary care physician’s detection of skin cancers. J Clin Aesthet Dermatol.2023;16(suppl 4):s16-17.
Newsletter
Stay up to date on recent advances in the multidisciplinary approach to cancer.