
Only in Hollywood can New Orleans stand in for Los Angeles while a suave crooner portrays a serious scientist.

Your AI-Trained Oncology Knowledge Connection!


Only in Hollywood can New Orleans stand in for Los Angeles while a suave crooner portrays a serious scientist.

The lead investigator in an evaluation of bevacizumab (Avastin) combined with sunitinib malate (Sutent) for renal cell carcinoma (RCC) said the project would be abandoned. FDA issued a product safety alert after Genentech, Avastin’s developer, reported serious complications in several patients enrolled in the phase I trial.

Polypharmacy, defined as concurrent use of several drugs, is not uncommon in the elderly and increases their risk of adverse drug reactions and interactions.[1] Besides adverse drug reactions and drug-drug interactions, other clinical sequelae of polypharmacy include nonadherence, increased risk of hospitalizations, and medication errors.

Bayer HealthCare Pharmaceuticals and Onyx Pharmaceuticals, Inc, recently announced that The New England Journal of Medicine published results of a phase III trial demonstrating that sorafenib (Nexavar) tablets decreased the absolute risk of death by 31% in patients with unresectable hepatocellular carcinoma (HCC) vs patients who received placebo. This represents a 44% improvement in median overall survival for patients treated with sorafenib.

There are a plethora of websites that hawk miracle cancer cures, luring consumers in with seductive testimonials of instant good health. To the average person, these sites are dubious at best. But for some cancer patients, the promise of an easy panacea veers on dangerous.

As a boy growing up in the Jim Crow South, LaSalle Leffall, Jr., MD, lifted himself above that era’s stifling segregation by embracing his father’s rock-solid credo: With a good education and hard work, combined with honesty and integrity, there are no boundaries.

A common conundrum that community oncologists face in their practices is whether to bill a first encounter with a new patient referred by another physician as a consultation or as a new patient visit. Making the distinction may seem like splitting hairs, but the Centers for Medicare and Medicaid Services (CMS) has very specific billing criteria on this issue.

Annually, adverse drug reactions (ADRs) result in costs of $3.6 billion and 140,000 deaths.1 Yet in 2005, only 15,107 reports of fatalities linked to potential drug toxicity were reported to the US Food and Drug Administration.2 This low number suggests that, despite significant morbidity and morality, ADRs remain underappreciated by clinicians. This is particularly troublesome when it comes to ADRs associated with oncology drugs.

Radiation is often considered immunosuppressive, an activity that is most likely a result of the complex interplay of hormesis and the abscopal effect. The abscopal effect, also called the “distant bystander” effect, is a paradoxical effect of radiation on cellular systems whereby local radiation may have an antitumor effect on tumors distant from the site of radiation.

CHICAGO-Sunday, June 1 marked the 15th annual Cancer Survivors’ Celebration and Walk, sponsored by the Robert H. Lurie Cancer Center of Northwestern University as part of National Cancer Survivors Day. More than 800 cancer survivors participated in the walk (see photograph).

This review focuses on discussing the efficacy and cardiotoxicity of anthracycline and trastuzumab combination regimens compared to nonanthracycline and trastuzumab regimens.

Among 185 patients with acute myeloid leukemia, 34 had RAS mutations and 22 received high-dose cytarabine after complete remission.

Long-term survivors of testicular cancer treated with cisplatin-based chemotherapy have evidence of endothelial injury and dysfunction, compared with those who did not receive chemotherapy, according to a University of Pennsylvania study (Cancer 112:1949-1953, 2008).

RCELONA-Colon cancer patients who received oral capecitabine (Xeloda) after surgery had significantly better 5-year survival rates than those given IV 5-FU/LV chemotherapy, according to a preplanned multivariate analysis of the X-ACT trial data presented at the 2008 World Congress on Gastrointestinal Cancer (Twelves et al: poster 0-033).

UVEN, Belgium-ThromboGenics NV and BioInvent International AB (Lund, Sweden) have entered into a license agreement with Roche for their jointly developed anticancer agent TB-403, a novel monoclonal antibody that blocks PIGF, one of the growth factors responsible for angiogenesis.

In May 2008, ONI reviewed an article by Herman Suit, MD, and colleagues in which they argued that randomized trials of proton beam therapy vs standard radiotherapy are not needed prior to a wider use of proton beam therapy. In an ONI reader poll, 81% disagreed with Dr. Suit, as does Dr. Robert Parker, of SUNY Stony Brook, in his letter below.


The authors of this article accomplished their goal to provide an overview of physical long-term / late effects. Similar to most available literature published since the Institute of Medicine report in November 2005, it provided a descriptive summary of the epidemiologic data. While vital to increasing the knowledge base of nurses on the frontlines, it provides little guidance as to how to change or improve practice.

In cancer treatment these days, the immediate-what needs to be done for the patient right now toward achieving long-term survival-is coupled with planning post-treatment surveillance, care, and support for patients who will likely survive their disease.

Drug is indicated for rescue of normal cells following high dose methotrexate administration for osteosarcoma. It is also indicated to diminish and counteract methotrexate toxicity if the drug is not effectively eliminated, or for inadvertent overdose of folic acid antagonists.

CHICAGO-This year’s roiling political contest took center stage at ASCO 2008 in a special session that reviewed the healthcare insurance reform proposals of Sen. John McCain and Sen. Barack Obama, the presumptive presidential nominees.

Matthew Ringel, MD, and colleagues have received an $11.9 million NCI grant for their research in thyroid cancer. The 5-year grant will fund the group’s work on genetics and signaling pathways in epithelial thyroid cancer. Dr. Ringel is co-director of the thyroid cancer unit at Ohio State University in Columbus.

The use of the term "futility" in cancer care has been prompted, in part, by increasing requests from patients for treatments thought to be ineffective as well as costly.[1] The appropriate role of chemotherapy near the end of life is a complex issue.[2]

Decision-making at the end of life is difficult, and it should be. Rather than face these time consuming and emotionally demanding discussions, doctors too often look to unsuitable conceptual models.

The article by Khatcheressian and colleagues addresses the important topic of futility in chemotherapy use. While extensive previous literature has addressed the use of futile treatment by oncologists, Khatcheressian and coauthors pose interesting perspectives on patient persistence in seeking futile treatment.
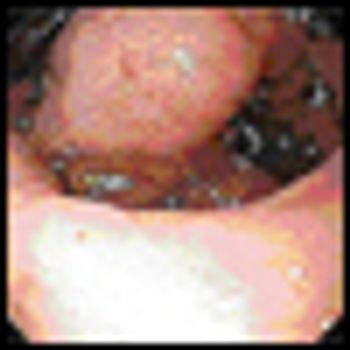
Our case illustrates the fact that MDS-associated GS can be treated palliatively with radiation and hypomethylating agents in an appropriate setting. With the growing geriatric patient population, effective treatment options are needed in this disease.

orafenib (Nexavar) tablets significantly improved overall survival by 47.3% (HR = 0.68; P = .014) in patients in the Asia-Pacific region with advanced hepatocellular carcinoma (HCC) vs those receiving placebo. Nexavar also significantly improved time to progression in these patients by 74% (HR = 0.57; P = .001).

The first phase III study of its kind has found that vaginal brachytherapy—in which a radioactive cylinder is inserted into the vagina—is as effective at preventing the recurrence of higher-risk endometrial cancer as external-beam radiation therapy, has fewer side effects, and results in a better quality of life for patients (abstract LBA5503).

There is a large musical instrument, a vibraphone, occupying a corner of Dr. Larry Norton’s office, but it doesn’t seem out of place. He is a serious musician, so serious in fact that in his youth he thought hard about a career in music.

CHICAGO-For breast cancer patients whose tumors have become resistant to available agents, restoring the sensitivity to treatment is an important goal. Preclinical studies have suggested that drugs that inhibit the mTOR protein kinase-which acts as a central regulator of tumor cell division, cell metabolism, and blood vessel growth-may be able to do so.