FDA Approves Penpulimab in Non-Keratinizing Nasopharyngeal Carcinoma
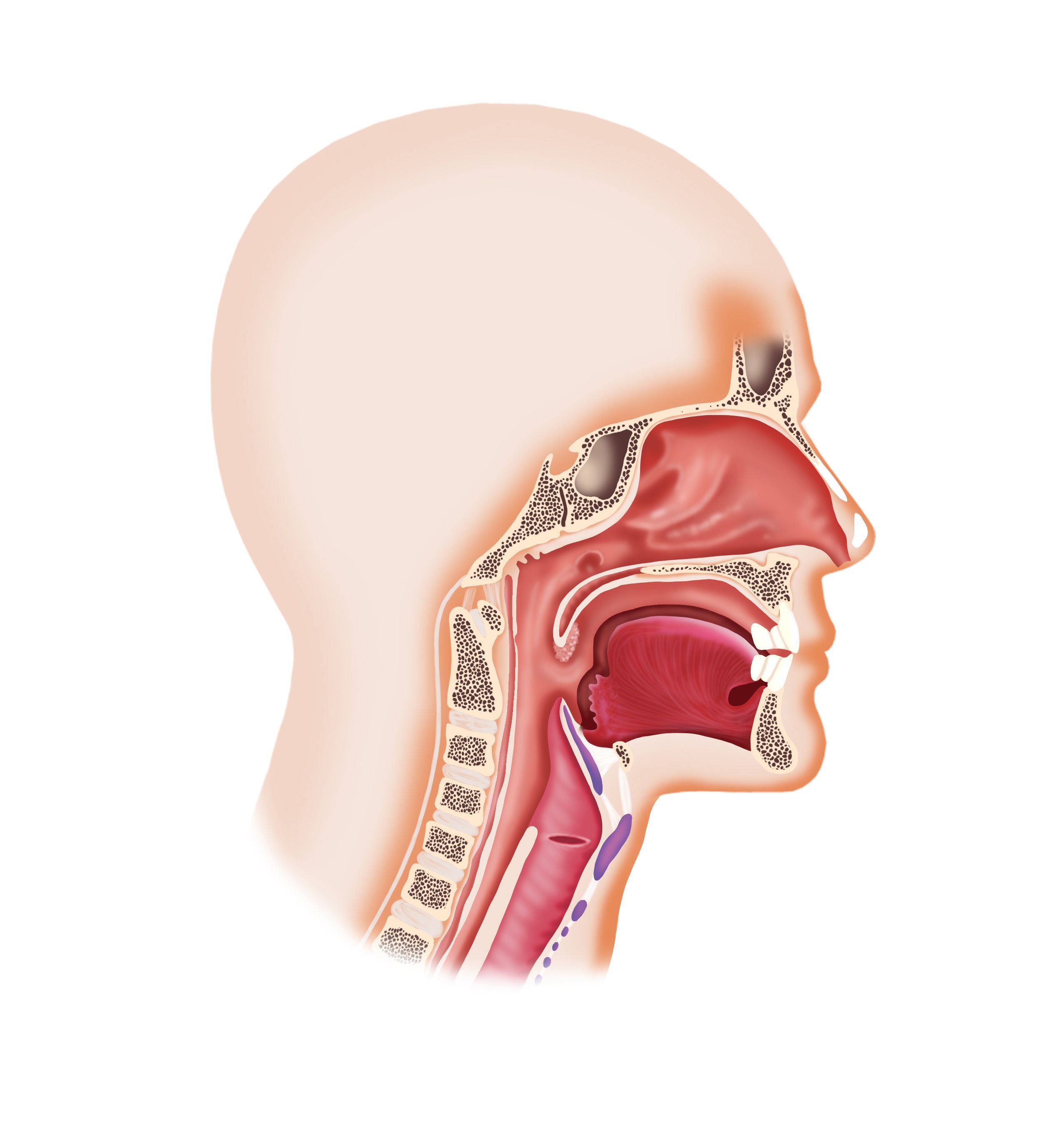
Data from the phase 3 AK105-304 study support the approval of penpulimab in this nasopharyngeal carcinoma population.
The FDA has approved penpulimab-kcqx (Anike) with cisplatin or carboplatin and gemcitabine as a first-line treatment for adult patients with recurrent or metastatic non-keratinizing nasopharyngeal carcinoma (NPC), according to a news release from the FDA. In addition, penpulimab was approved as a single-agent therapy for adult patients with metastatic non-keratinizing NPC experiencing disease progression on or after platinum-based chemotherapy and one additional line of therapy.
Support for the FDA decision came from the phase 3 AK105-304 trial (NCT04974398), which evaluated the efficacy of penpulimab vs placebo with cisplatin or carboplatin and gemcitabine. Therein, the median progression-free survival (PFS) was 9.6 months (95% CI, 7.1-12.5) in the investigational arm vs 7.0 months (95% CI, 6.9-7.3) in the placebo arm (HR, 0.45; 95% CI, 0.33-0.62; P <.0001). The 12-month PFS rates were 31% and 11%, respectively. Although overall survival (OS) results were immature at the time of data cutoff, no detrimental trend was observed with penpulimab.
Additionally, the efficacy of penpulimab monotherapy was evaluated in the single-arm phase 2 AK105-202 trial (NCT03866967) in 125 patients with unresectable or metastatic non-keratinizing NPC who experienced disease progression on or after platinum-based chemotherapy and one additional line of therapy.
Immune-mediate adverse effects (AEs) related to penpulimab treatment included pneumonitis, colitis, hepatitis, endocrinopathies, nephritis with renal dysfucntion, and skin toxicities. AEs occurring in more than 20% of patients treated with penpulimab with chemotherapy included nausea, vomiting, hypothyroidism, constipation, decreased appetite, decreased weight, cough, COVID-19 infection, fatigue, rash, and pyrexia. Additionally, the most common AEs for penpulimab monotherapy included hypothyroidism and musculoskeletal pain. Fatal AEs occurred in 1% of patients, including individual cases of pneumonitis, septic shock, colitis, and hepatitis.
The recommended dose of penpulimab with cisplatin or carboplatin and gemcitabine is 200 mg once every 3 weeks until progression, unacceptable toxicity, or for a maximum of 24 months. The recommended single-agent dose for previously treated NPC is 200 mg biweekly until progression or unacceptable toxicity for a maximum of 24 months.
Reference
FDA approves penpulimab-kcqx for non-keratinizing nasopharyngeal carcinoma. News release. FDA. April 23, 2025. Accessed April 23, 2025. https://tinyurl.com/yy29zm3u
Newsletter
Stay up to date on recent advances in the multidisciplinary approach to cancer.