Messenger RNA Vaccines: Beckoning of a New Era in Cancer Immunotherapy
A review of mRNA vaccine technology by ONCOLOGY® editorial board member Mehmet Sitki Copur, MD, FACP, explains the basis for progress in this field and reviews a number of new trials using this technology.
Copur is a medical oncologist/hematologist and Medical Director of Oncology at Morrison Cancer Center, Mary Lanning Healthcare in Hastings, Nebraska, and is a professor at the University of Nebraska Medical Center adjunct faculty. He is also an Editorial Advisory Board member at ONCOLOGY®.

ABSTRACT
Messenger RNA (mRNA) vaccines are a relatively new class of vaccines. They combine the potential of mRNA to encode for almost any protein with an excellent safety profile and a flexible production process. During the last decade, the mRNA vaccine approach has been increasingly recognized and viewed as a versatile tool for the development of new innovative therapeutics not only in infectious disease settings but also in cancer. mRNA vaccines traditionally consist of a messenger RNA synthesized by in vitro transcription using a bacteriophage RNA polymerase and a template DNA that encodes the antigen(s) of interest. Once administered and internalized by host cells, the mRNA transcripts are translated directly in the cytoplasm of the cell. The resulting antigens are presented to the immune system cells to stimulate an immune response. Dendritic cells (DCs) can be utilized as a carrier by delivering tumor-associated antigen mRNAs or total tumor RNA to their cytoplasm; then, the mRNA-loaded DCs can be delivered to the host to elicit a specific immune response. Recently, 2 mRNA vaccines were approved for the first time for human use—to prevent COVID-19 infection—bringing excitement for the future possibilities of this approach for cancer immunotherapy as well as for preventing other infectious diseases.
Oncology (Williston Park). 2021;35(4):190-198.
DOI: 10.46883/ONC.2021.3504.0198
Introduction
Messenger RNA (mRNA) vaccines have generated significant excitement not only for their potential to complement or even replace traditional vaccines against infectious diseases, but also for their potential use in noninfectious diseases, including cancer. Nucleic acid therapeutics have emerged as promising alternatives to conventional vaccine approaches. Exploitation of mRNA, the intermediate step between the protein-encoding DNA and the protein manufactured by ribosomes, has proven to be a favorable alternative to conventional vaccines. In this approach, a linear DNA template and a T7, a T3, or an Sp6 phage RNA polymerase are used in vitro to design the mRNA containing 5' and 3' untranslated regions (UTRs). The resulting product contains an open reading frame that encodes the protein of interest, flanking UTRs, a 5' cap, and a poly(A) tail; this tail resembles the fully processed mature mRNA molecule that naturally occurs in the cytoplasm of eukaryotic cells (FIGURE 1).1 Successful cytosolic delivery of mRNA results in synthesis of the encoded protein, which undergoes posttranslational modifications and results in a properly folded, fully functional product that is delivered to the correct cellular compartments for proper presentation or function.
FIGURE 1. mRNA Vaccine Design
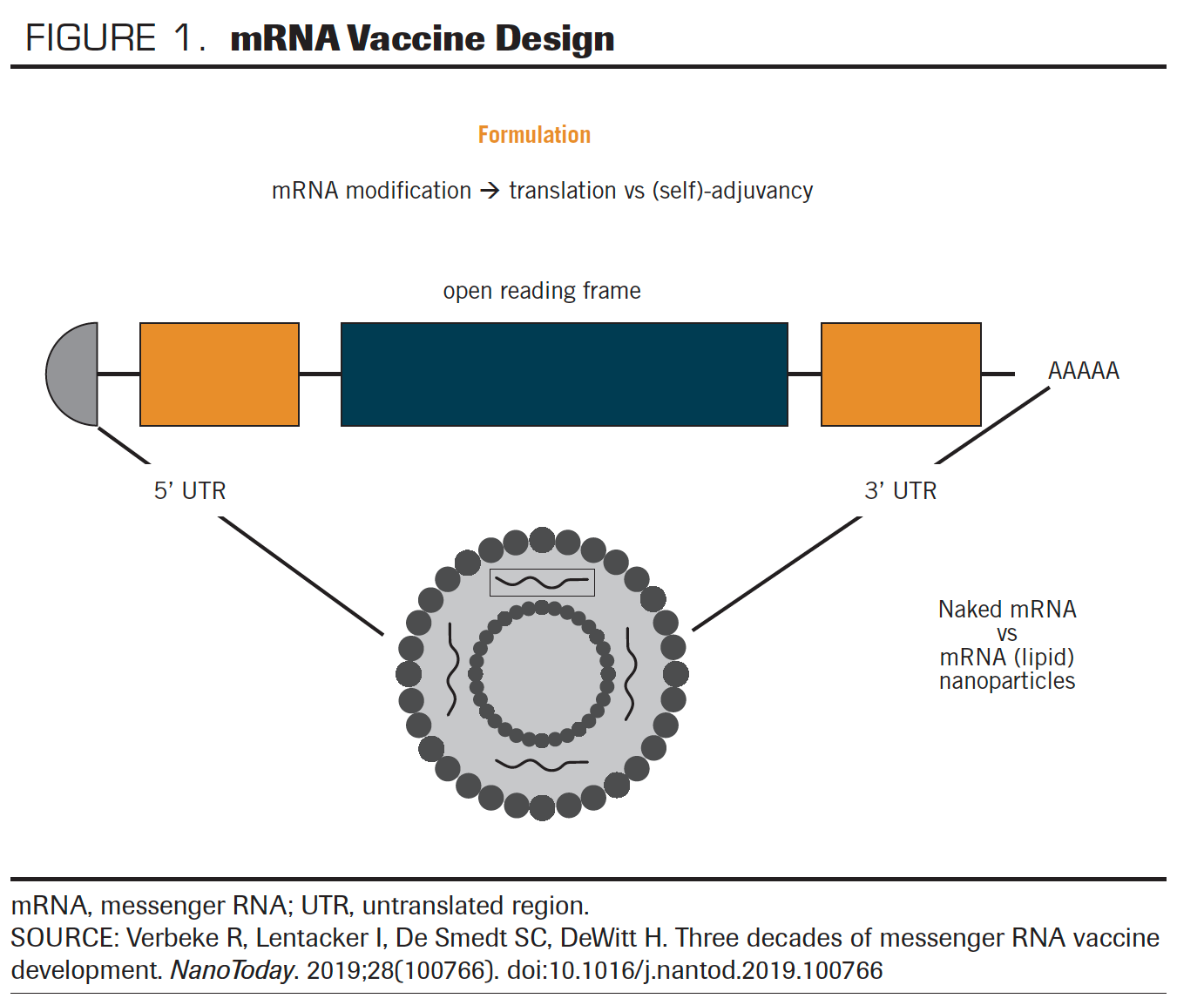
In vitro transcribed mRNA is ultimately degraded by normal cellular physiological processes.2-4 Because the target for mRNA vaccines is the cytoplasm of the cell, they are easier to deliver, and cytosolic mRNA has no interaction with the genome.5 The small piece of genetic information mRNA vaccine carries is expressed only transiently, until it is degraded. mRNA can encode multiple proteins that possess very different chemical and physical properties without major changes in its physiochemical properties. mRNAs can theoretically be used to produce any protein, and doing so is a much more simple and cost-effective way of manufacturing the proteins.
While the concept of possibly using mRNA vaccines against infectious diseases, genetic disorders, and cancer has been around for decades, until now no vaccines using this technology have received FDA approval for human use. Recently, 2 phase 3 trials led to the first FDA approvals of mRNA-based vaccines, and this marks the beginning of a new era not only for the infectious disease field but also for oncology.
Background
In an early proof-of-concept study, RNA and DNA expression vectors that contained genes for chloramphenicol acetyltransferase, luciferase, and beta-galactosidase were separately injected into mouse skeletal muscle; expression of all the proteins was later readily detected. The extent of expression from both the RNA and DNA constructs was comparable with that obtained from
fibroblasts transfected in vitro under optimal conditions.6
Despite its revolutionary promise, though, mRNA work has been hampered by several challenges. These have included the instability of mRNA molecules, leading them to degrade quickly inside the cell; the inability of mRNAs to produce high levels of proteins; and mRNA`s own immunogenicity, which triggers an immune response independent of the desired immune response, that it attempts to produce to the protein it encodes.
DNA and RNA can stimulate the mammalian innate immune system through activation of Toll-like receptors (TLRs). Observation of lack of immunostimulatory effect of DNAs containing methylated CpG motifs and selected nucleosides in naturally occurring methylated or otherwise modified RNAs led to a discovery that was reported in a seminal paper by Kariko et al. They showed that RNA creates signals through human TLR3, TLR7, and TLR8; however, the incorporation of modified nucleosides m5C, m6A, m5U, or s2U, or of pseudouridine, ablates this activity. Dendritic cells (DCs) exposed to such modified RNA express significantly fewer cytokines and activation markers than those treated with unmodified RNA. This discovery—that modified synthetic nucleosides could both increase protein production from mRNA and suppress the immune system`s recognition and degradation of mRNA—helped mRNA vaccine technology evolve further.7
Under physiological conditions, RNA molecules are highly unstable and very sensitive to catalytic hydrolysis by ubiquitous ribonucleases.8 One of the main challenges for the clinical use of mRNA vaccines has been the difficulty in intracellularly delivering the mRNA. Naked mRNA, delivered by itself, is rapidly degraded after injection. Several mRNA delivery approaches have been tested, using substances including modified RNAs, RNA conjugates, viral vectors, microparticles, and, most successfully, lipid nanoparticles.9-11 Lipid nanoparticles are multicomponent lipid systems composed of an ionizable lipid, a phospholipid, cholesterol, and a polyethylene glycol (PEG)-lipid. The ionizable lipid facilitates the mRNA’s formation of a core structure, while helper lipids (phospholipid and cholesterol) envelop the lipid-mRNA complex, and the PEG-lipid protects the nanoparticle shell. Lipid nanoparticles have been the most promising innovation in this field.12
FIGURE 2. In Vivo Delivery of mRNA Vaccine
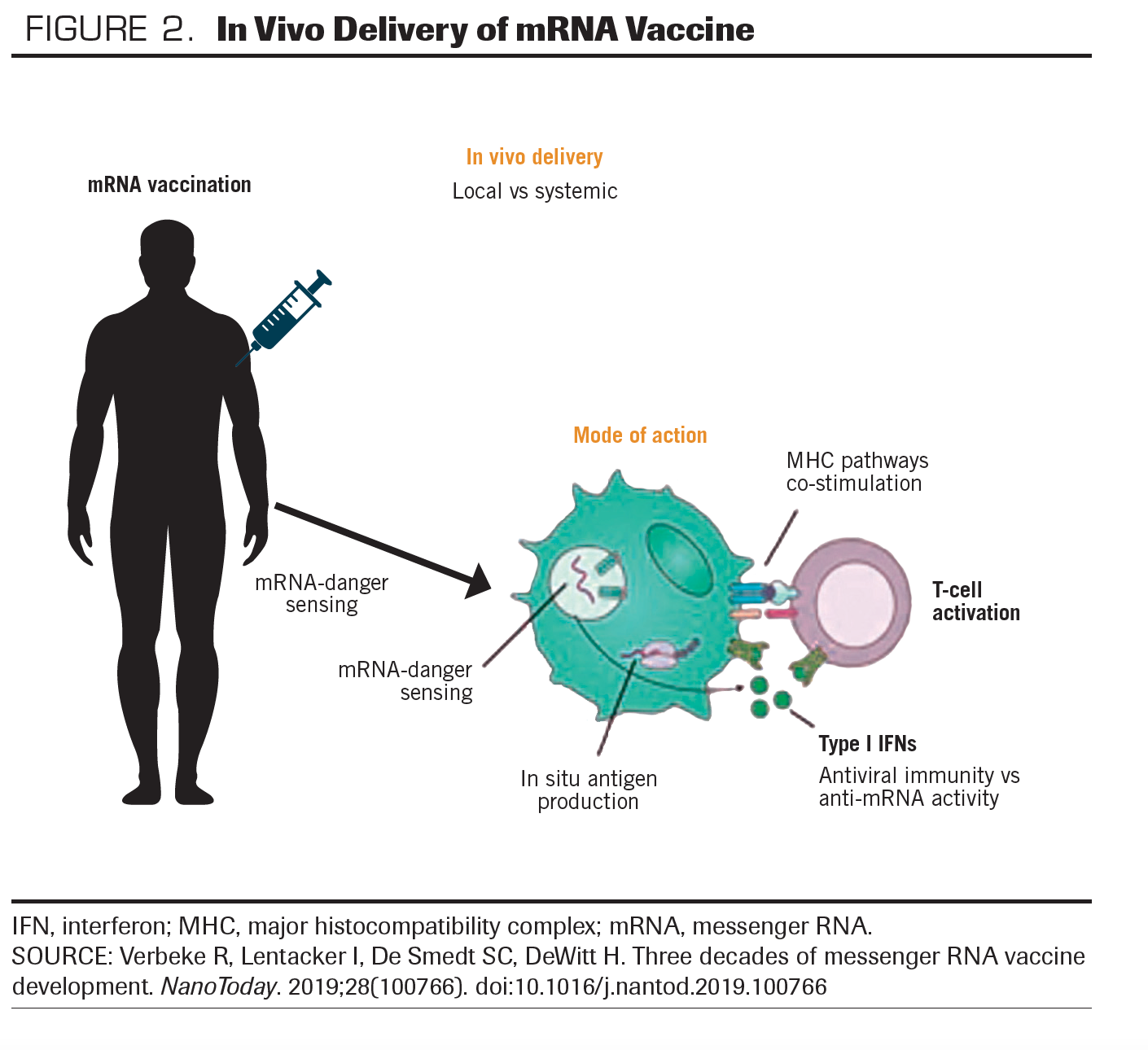
For efficacy, adequate in vivo delivery of exogenous mRNA is crucial (FIGURE 2). Uptake of mRNA may depend on the physicochemical properties of the mRNA complexes and the cell type. Two main strategies have been utilized for the in vivo delivery of mRNAs. One such approach involves loading mRNA into DCs ex vivo, followed by reinfusion of the transfected cells. This allows precise control of the cellular target and transfection efficiency, but it is expensive and labor intensive. The DC approach elicits a predominantly cell-mediated immune response; it has been explored primarily in cancer treatment.
The second approach is direct parenteral injection of mRNA. This is relatively quick and cost effective, but it may not be as precise and efficient in specific cell delivery. The systemic delivery of vaccine antigens can be facilitated by using RNA-lipoplexes. The lipoplexes protect RNA from extracellular ribonucleases; they mediate the efficient uptake and expression of the encoded antigen.13,14 Recently, highly efficient and nontoxic RNA carriers have been developed that allow prolonged antigen expression in vivo.15,16 Some vaccine formulations contain novel adjuvants, while others elicit potent responses in the absence of known adjuvants.
Lipid Nanoparticle Approach for mRNA Vaccines
mRNA has a number of benefits over traditional treatments, but its use as a therapeutic molecule has been challenging due to its chemical instability and susceptibility to hydrolysis, catalyzed by ubiquitous RNA nucleases. However, recent advances in nonviral delivery systems and the development of novel effective transfecting nanomaterials have brought some effective resolutions to these challenges.
The encapsulation of mRNA into a carrier is necessary to protect it from extracellular degradation and to stimulate its cellular uptake and endosomal escape.17,18 Lipid-based delivery systems of lipoplexes have some drawbacks, including high instability, relatively low transfection efficiency, and poor customizable composition, so the earlier use of these systems15,19 has been replaced by lipid nanoparticles. Lipid nanoparticles have demonstrated superior stability and structural plasticity as well as enhanced gene delivery.18,20 A lipid nanoparticle is composed of pH-responsive lipids; neutral helper lipids, such as zwitterionic lipid and/or sterol lipid (ie, cholesterol), to stabilize the lipid bilayer of the lipid nanoparticle; and a PEG-lipid to improve the colloidal stability in biological environments.18,21 The application of lipid-based nanotechnology delivery systems is now enabling the integration of mRNA-based technology with many preexisting anticancer immunotherapeutic approaches, including therapeutic vaccines, monoclonal antibodies, and chimeric antigen receptor T-cell therapy. A deeper understanding of key parameters, such as hydrophobicity and fusogenicity determining the transfection efficiency of the formulation and discoveries of how they can be modulated by varying the lipidic composition or through the introduction of novel lipids, will further enhance the development of better formulations. More research is needed to understand the reasons behind the low mRNA transfection efficiency.
mRNA Vaccines Against Cancer
While mRNA technology is a relatively new and novel approach in cancer vaccine development, vaccines in general have previously been conceptually considered in cancer immunotherapy, by utilizing viral or bacterial vaccines. fact, the concept goes as far back as 1891, when Dr. William Coley made the first attempt to stimulate the immune system to improve a patient’s condition: He used intratumoral injections of inactivated Streptococcus pyogenes and Serratia marcescens, a mixture now known as Coley’s toxin.22 The bacillus Calmette-Guérin (BCG), a bacterium that functions similarly to Coley’s toxin, is currently used to treat superficial bladder cancer.23,24
However, the clinical translation of cancer vaccines into efficacious therapies that could be used widely has been challenging for decades. The 2 very first US FDA vaccine approvals in cancer were for prophylactic use: hepatitis B virus vaccine and human papillomavirus vaccine. The first therapeutic cancer vaccine was not available until 2010, with approval of an immune cell–based vaccine, sipuleucel-T, for prostate cancer refractory to hormone therapy.25
Over the past decades, numerous diverse therapeutic cancer vaccination strategies have been under development. Recent human-use approval of the 2 mRNA vaccines for COVID-19 may mark the end of the beginning of our quest for mRNA vaccine immunotherapy of cancer.26,27 The early proof-of-concept studies that provided the evidence for the feasibility of the mRNA vaccine approach were published more than 2 decades ago.28,29 Since then, numerous preclinical and clinical research teams have been exploring the viability of this approach. Most cancer vaccines seek to stimulate cell-mediated responses, such as those from cytotoxic T lymphocytes, that are capable of clearing or reducing tumor burden.30 Cancer vaccines can be designed to target tumor-associated antigens that are preferentially expressed in cancerous cells. Growth-associated factors, or antigens that are unique to malignant cells due to somatic mutations, can be targeted in the design of mRNA cancer vaccines.31 While the simplicity of mRNA vaccines greatly reduces the difficulties generally associated with the production of biological vaccines, the route of administration and delivery format greatly influence outcomes. A variety of mRNA cancer vaccines have been studied that utilize the 2 main delivery strategies: direct injection and the DC approach.
Direct Injection mRNA Approach
Direct injection of mRNA can be accomplished through common delivery routes, such as intradermal, subcutaneous, intranasal, and intramuscular, as well as some unconventional routes, such as intranodal, intrasplenic, intratumoral, and intravenous. Systemic administration of mRNAs has been challenging, due to concerns about aggregation with serum proteins and rapid extracellular degradation, which has led to formulations with carrier molecules. Numerous delivery formulations have been developed to facilitate mRNA uptake, increase protein translation, and protect mRNA from RNAases, as discussed above.
The intradermal route of delivery has been widely used for mRNA cancer vaccines. The presence of a variety of antigen-presenting cells in the skin makes it an ideal site for immunogen delivery.32 In several mouse tumor models of various cancers, including fibrosarcoma, melanoma, and prostate cancer, vaccines delivered intradermally have been shown to produce antitumor responses.32-34
Intranasal administration, a needle-free, noninvasive delivery model, has also been shown to enable rapid antigen uptake by DCs. In mouse tumor models, intranasal delivery of mRNA complexed with lipid nanoparticles has been shown to induce prophylactic and therapeutic antitumor immunity.35
A rather unconventional but efficient mRNA vaccine delivery approach has been the intranodal administration of unprotected mRNA, directly injected into lymphoid tissue. Intranodally injected mRNAs can stimulate potent prophylactic or therapeutic antitumor T-cell responses.36,37 Similar efficacy has been shown by intrasplenic delivery of mRNA melanoma vaccine in mouse tumor models.38 Intranodal injection of naked mRNA encoding tumor-associated antigens in patients with melanoma (NCT01684241) and with hepatocellular carcinoma (EudraCT: 2012-005572-34) is being studied in human clinical trials.
Finally, another unconventional direct delivery method that has been shown to be effective is intratumoral mRNA delivery. Here, intratumoral administration of mRNAs that do not encode tumor-associated antigens seems to stimulate tumor-specific immunity through the intrinsic immunogenic properties of mRNA.39-41
Dendritic Cell mRNA Approach
DCs are the professional antigen-presenting cells of our immune system. They can take up, process, and present antigens to T cells. mRNA is a polyvalent molecule with an attractive safety profile and the ability to induce transient but sufficiently long and high protein expression without the risk of integrating into the host genome. These characteristics make it an attractive vector to deliver tumor antigens to DCs.
The interest in using mRNA as a means of loading tumor antigens onto DCs goes back to the late 1990s, when initial attempts were based on passive pulsing. They relied on the ability of DCs to take up the mRNA, translate it into proteins, then process it into peptides and finally present it to CD8+ T cells.32,42,43 To increase the efficiency of uptake, investigators have studied several strategies, including electroporation, nucleofection, lipofection, and, more recently, sonoporation.44-47
A variety of immune-regulatory proteins in the form of mRNA-encoded adjuvants or co-stimulatory molecules have been shown to increase the potency of DC cancer vaccines. These molecules include CD83, tumor necrosis factor receptor superfamily member 4 (TNFRSF4; also known as OX40), and 4-1BB ligand. Electroporation of DCs with these mRNAs have resulted in a substantial increase in the immune stimulatory activity of DCs.48-51 Further, the use of mRNA-encoded proinflammatory cytokines, such as IL-12, or associated molecules has been shown to enhance and potentiate the efficacy of immune response.52-55 In a phase 2 trial, the combination of a mRNA DC vaccine approach with an immune checkpoint inhibitor has shown durable tumor response.56
TABLE. Current Cancer Clinical Trials of mRNA Vaccine
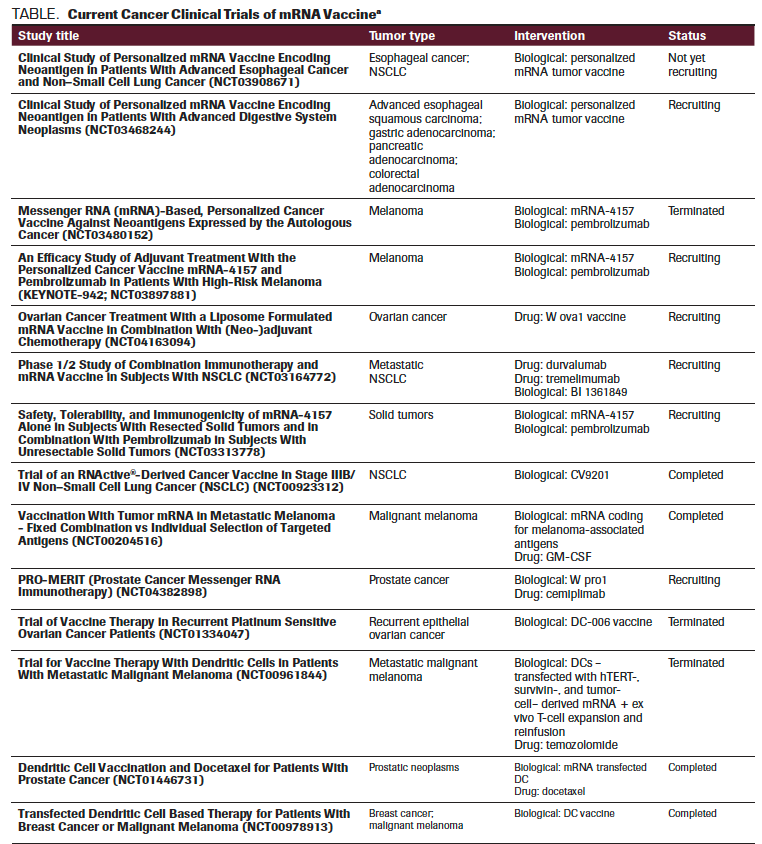
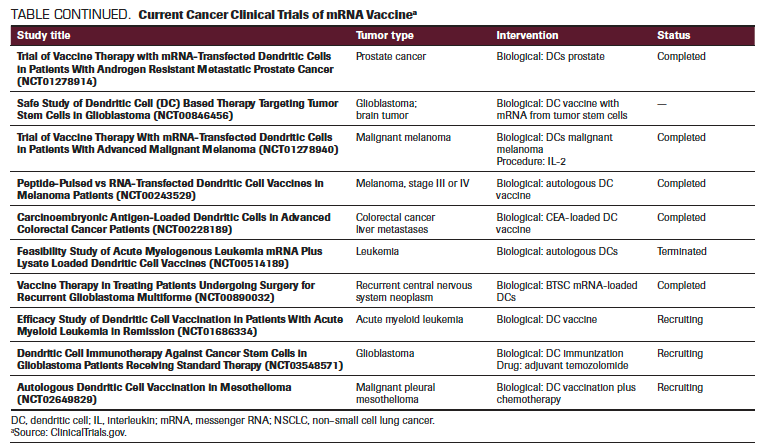
TABLE CONTINUED. Current Cancer Clinical Trials of mRNA Vaccine
Conclusions
Within the past 2 decades, major achievements in the field of mRNA vaccines have created a safe and attractive treatment option for a broad range of diseases, including cancer. Their simplicity, high potency, capacity for rapid development, and potential for low-cost manufacture and safe administration all make mRNA vaccines a very promising alternative to conventional vaccine approaches. Recent improvements in mRNA vaccines to increase protein translation, modulate innate and adaptive immunogenicity, and improve delivery are very encouraging. The combination of mRNA vaccination with adjunctive therapies, such as traditional chemotherapy, radiotherapy, and immune checkpoint inhibitors, may contribute further to the approach’s benefit in oncology. Challenges may include scaling up good manufacturing practice production, establishing regulations, further documenting safety, and increasing efficacy. Clinical studies in various cancer types are moving forward, and promising results with favorable clinical outcomes are eagerly awaited (TABLE). Future research should also focus on interpreting the immune pathways activated by various mRNA vaccine platforms and try to improve current approaches based on these mechanisms. Recent approval of 2 mRNA vaccines for COVID-19 for human use opens the gateway for many future applications of this novel treatment approach.
Financial Disclosure: The authors have no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
References
1. Pardi N, Muramatsu H, Weissman D, Karikó K. In vitro transcription of long RNA containing modified nucleosides. Methods Mol Biol. 2013;969:29-42. doi:10.1007/978-1-62703-260-5_2
2. Liu MA. Immunologic basis of vaccine vectors. Immunity. 2010;33(4):504-515. doi:10.1016/j.immuni.2010.10.004
3. Hilleman MR. Recombinant vector vaccines in vaccinology. Dev Biol Stand. 1994;82:3-20.
4. Deering RP, Kommareddy S, Ulmer JB, Brito LA, Geall AJ. Nucleic acid vaccines: prospects for non-viral delivery of mRNA vaccines. Expert Opin Drug Deliv. 2014;11(6):885-899. doi:10.1517/17425247.2014.901308
5. Jäschke A, Helm M. RNA sex. Chem Biol. 2003;10(12):1148-1150. doi:10.1016/j.chembiol.2003.12.003
6. Wolff JA, Malone RW, Williams P, et al. Direct gene transfer into mouse muscle in vivo. Science. 1990;247(4949 pt 1):1465-1468. doi:10.1126/science.1690918
7. Karikó K, Buckstein M, Ni H, Weissman D. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity. 2005;23(2):165-175. doi:10.1016/j.immuni.2005.06.008
8. Sorrentino S. Human extracellular ribonucleases: multiplicity, molecular diversity and catalytic properties of the major RNase types. Cell Mol Life Sci. 1998;54(8):785-794. doi:10.1007/s000180050207
9. Chira S, Jackson CS, Oprea I, et al. Progresses towards safe and efficient gene therapy vectors. Oncotarget. 2015;6(31):30675-30703. doi:10.18632/oncotarget.5169
10. Ku SH, Jo SD, Lee YK, Kim K, Kim SH. Chemical and structural modifications of RNAi therapeutics. Adv Drug Deliv Rev. 2016;104:16-28. doi:10.1016/j.addr.2015.10.015
11. Lundstrom K. Alphaviruses in gene therapy. Viruses. 2009;1(1):13-25. doi:10.3390/v1010013
12. Rizk M, Tüzmen Ş. Update on the clinical utility of an RNA interference-based treatment: focus on Patisiran. Pharmgenomics Pers Med. 2017;10:267-278. doi:10.2147/PGPM.S87945
13. Benteyn D, Heirman C, Bonehill A, Thielemans K, Breckpot K. mRNA-based dendritic cell vaccines. Expert Rev Vaccines. 2015;14(2):161-176. doi:10.1586/14760584.2014.957684
14. Kranz LM, Diken M, Haas H, et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature. 2016;534(7607):396-401. doi:10.1038/nature18300
15. Pardi N, Tuyishime S, Muramatsu H, et al. Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes. J Control Release. 2015;217:345-351. doi: 10.1016/j.jconrel.2015.08.007
16. Bahl K, Senn JJ, Yuzhakov O, et al. Preclinical and clinical demonstration of immunogenicity by mRNA vaccines against H10N8 and H7N9 influenza viruses. Mol Ther. 2017;25(6):1316-1327. doi:10.1016/j.ymthe.2017.03.035
17.Guan S, Rosenecker J. Nanotechnologies in delivery of mRNA therapeutics using nonviral vector-based delivery systems. Gene Ther. 2017;24(3):133-143. doi:10.1038/gt.2017.5
18. Guevara ML, Jilesen Z, Stojdl D, Persano S. Codelivery of mRNA with α-galactosylceramide using a new lipopolyplex formulation induces a strong antitumor response upon intravenous administration. ACS Omega. 2019;4(8):13015-13026. doi:10.1021/acsomega.9b00489
19. Felgner PL, Ringold GM. Cationic liposome-mediated transfection. Nature. 1989;337(6205):387-388. doi:10.1038/337387a0
20. Xue HY, Guo P, Wen W-C, Wong HL. Lipid-based nanocarriers for RNA delivery. Curr Pharm Des. 2015;21(22):3140-3147. doi:10.2174/1381612821666150531164540
21. Cullis PR, Hope MJ. Lipid nanoparticle systems for enabling gene therapies. Mol Ther. 2017;25(7):1467-1475. doi: 10.1016/j.ymthe.2017.03.013
22. McCarthy EF. The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop J. 2006;26:154-158.
23. Lamm DL, Blumenstein BA, Crawford ED, et al. A randomized trial of intravesical doxorubicin and immunotherapy with bacille Calmette-Guérin for transitional-cell carcinoma of the bladder. N Engl J Med. 1991;325(17):1205-1209. doi:10.1056/NEJM199110243251703
24. van der Meijden APM, Sylvester RJ, Oosterlinck W, Hoeltl W, Bono AV, EORTC Genito-Urinary Tract Cancer Group. Maintenance Bacillus Calmette-Guerin for Ta T1 bladder tumors is not associated with increased toxicity: results from a European Organisation for Research and Treatment of Cancer Genito-Urinary Group phase III trial. Eur Urol. 2003;44(4):429-434. doi:10.1016/s0302-2838(03)00371-3
25. Cheever MA, Higano CS. PROVENGE (Sipuleucel-T) in prostate cancer: the first FDA-approved therapeutic cancer vaccine.Clin Cancer Res. 2011;17(11):3520-3526. doi:10.1158/1078-0432.CCR-10-3126
26. Baden LR, El Sahly HM, Essink B, et al; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403-416. doi:10.1056/NEJMoa2035389
27. Polack FP, Thomas SJ, Kitchin N; C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. doi:10.1056/NEJMoa2034577
28.Conry RM, LoBuglio AF, Wright M, et al. Characterization of a messenger RNA polynucleotide vaccine vector. Cancer Res. 1995;55(7):1397-1400.
29. Boczkowski D, Nair SK, Snyder D, Gilboa E. Dendritic cells pulsed with RNA are potent antigen-presenting cells in vitro and in vivo. J Exp Med. 1996;184(2):465-472. doi:10.1084/jem.184.2.465
30. Coulie PG, Van den Eynde BJ, van der Bruggen P, Boon T. Tumour antigens recognized by T lymphocytes: at the core of cancer immunotherapy. Nat Rev Cancer. 2014;14(2):135-146. doi:10.1038/nrc3670
31. Vigneron N. Human tumor antigens and cancer immunotherapy. Biomed Res Int. 2015;2015:948501. doi:10.1155/2015/948501
32. Clausen BE, Stoitzner P. Functional specialization of skin dendritic cell subsets in regulating T cell responses. Front Immunol. 2015;6:534. doi:10.3389/fimmu.2015.00534
33. Granstein RD, Ding W, Ozawa H. Induction of anti-tumor immunity with epidermal cells pulsed with tumor-derived RNA or intradermal administration of RNA. J Invest Dermatol. 2000;114(4):632-636. doi:10.3389/fimmu.2015.00534
34. Fotin-Mleczek M, Duchardt KM, Lorenz C, et al. Messenger RNA-based vaccines with dual activity induce balanced TLR-7 dependent adaptive immune responses and provide antitumor activity. J Immunother. 2011;34(1):1-15. doi: 10.1097/CJI.0b013e3181f7dbe8
35. Weide B, Pascolo S, Scheel B, et al. Direct injection of protamine-protected mRNA: results of a phase 1/2 vaccination trial in metastatic melanoma patients. J Immunother. 2009;32(5):498-507. doi:10.1097/CJI.0b013e3181a00068
36. Phua KKL, Staats HF, Leong KW, Nair SK. Intranasal mRNA nanoparticle vaccination induces prophylactic and therapeutic anti-tumor immunity. Sci Rep. 2014;4:5128. doi:10.1038/srep05128
37. Diken M, Kreiter S, Selmi A, et al. Selective uptake of naked vaccine RNA by dendritic cells is driven by macropinocytosis and abrogated upon DC maturation. Gene Ther. 2011;18(7):702-708. doi:10.1038/gt.2011.17
38. Kreiter S, Selmi A, Diken M, et al. Intranodal vaccination with naked antigen-encoding RNA elicits potent prophylactic and therapeutic antitumoral immunity. Cancer Res. 2010;70(22):9031-9040. doi:10.1158/0008-5472.CAN-10-0699
39. Zhou WZ, Hoon DS, Huang SK, et al. RNA melanoma vaccine: induction of antitumor immunity by human glycoprotein 100 mRNA immunization. Hum Gene Ther. 1999;10(16):2719-2724. doi:10.1089/10430349950016762
40. Scheel B, Aulwurm S, Probst J, et al. Therapeutic anti-tumor immunity triggered by injections of immunostimulating single-stranded RNA. Eur J Immunol. 2006;36(10):2807-2816. doi:10.1002/eji.200635910
41. Van der Jeught K, Joe PT, Bialkowski L, et al. Intratumoral administration of mRNA encoding a fusokine consisting of IFN-β and the ectodomain of the TGF-β receptor II potentiates antitumor immunity. Oncotarget. 2014;5(20):10100-10113. doi:10.18632/oncotarget.2463
42. Van Lint S, Renmans D, Broos K, et al. Intratumoral delivery of TriMix mRNA results in T-cell activation by cross-presenting dendritic cells. Cancer Immunol Res. 2016;4(2):146-156. doi:10.1158/2326-6066.CIR-15-0163
43. Nair SK, Boczkowski D, Morse M, Cumming RI, Lyerly HK, Gilboa E. Induction of primary carcinoembryonic antigen (CEA)-specific cytotoxic T lymphocytes in vitro using human dendritic cells transfected with RNA. Nat Biotechnol. 1998;16(4):364-369. doi:10.1038/nbt0498-364
44. Galluzzi L, Senovilla L, Vacchelli E, et al. Trial watch: dendritic cell-based interventions for cancer therapy. Oncoimmunology. 2012;1(7):1111-1134. doi:10.4161/onci.21494
45. Van Tendeloo VF, Ponsaerts P, Lardon F, et al. Highly efficient gene delivery by mRNA electroporation in human hematopoietic cells: superiority to lipofection and passive pulsing of mRNA and to electroporation of plasmid cDNA for tumor antigen loading of dendritic cells. Blood. 2001;98(1):49-56. doi:10.1182/blood.v98.1.49
46. Tuyaerts S, Noppe SM, Corthals J, et al. Generation of large numbers of dendritic cells in a closed system using Cell Factories. J Immunol Methods. 2002;264(1-2):135-151. doi:10.1016/s0022-1759(02)00099-6
47. Melhem NM, Gleason SM, Liu XD, Barratt-Boyes SM. High-level antigen expression and sustained antigen presentation in dendritic cells nucleofected with wild-type viral mRNA but not DNA. Clin Vaccine Immunol. 2008;15(9):1337-1344. doi:10.1128/CVI.00154-08
48. De Temmerman M-L, Dewitte H, Vandenbroucke RE, et al. mRNA-Lipoplex loaded microbubble contrast agents for ultrasound-assisted transfection of dendritic cells. Biomaterials. 2011;32(34):9128-9135. doi:10.1016/j.biomaterials.2011.08.024
49. De Keersmaecker B, Heirman C, Corthals J, et al. The combination of 4-1BBL and CD40L strongly enhances the capacity of dendritic cells to stimulate HIV-specific T cell responses. J Leukoc Biol. 2011;89(6):989-999. doi:10.1189/jlb.0810466
50. Dannull J, Nair S, Su Z, et al. Enhancing the immunostimulatory function of dendritic cells by transfection with mRNA encoding OX40 ligand. Blood. 2005;105(8):3206-3613. doi:10.1182/blood-2004-10-3944
51. Aerts-Toegaert C, Heirman C, Tuyaerts S, et al. CD83 expression on dendritic cells and T cells: correlation with effective immune responses. Eur J Immunol. 2007;37(3):686-695. doi:10.1002/eji.200636535
52. Grünebach F, Kayser K, Weck MM, Müller MR, Appel S, Brossart P. Cotransfection of dendritic cells with RNA coding for HER-2/neu and 4-1BBL increases the induction of tumor antigen specific cytotoxic T lymphocytes. Cancer Gene Ther. 2005;12(9):749-756. doi:10.1038/sj.cgt.7700842
53. Bontkes HJ, Kramer D, Ruizendaal JJ, Meijer CJLM, Hooijberg E. Tumor associated antigen and interleukin-12 mRNA transfected dendritic cells enhance effector function of natural killer cells and antigen specific T-cells. Clin Immunol. 2008;127(3):375-384. doi:10.1016/j.clim.2008.02.001
54. Bontkes HJ, Kramer D, Ruizendaal JJ, et al. Dendritic cells transfected with interleukin-12 and tumor-associated antigen messenger RNA induce high avidity cytotoxic T cells. Gene Ther. 2007;14(4):366-375. doi: 10.1038/sj.gt.3302874
55. Bonehill A, Tuyaerts S, Van Nuffel AMT, et al. Enhancing the T-cell stimulatory capacity of human dendritic cells by co-electroporation with CD40L, CD70 and constitutively active TLR4 encoding mRNA. Mol Ther. 2008;16(6):1170-1180. doi:10.1038/mt.2008.77
56. Wilgenhof S, Corthals J, Heirman C, et al. Phase II study of autologous monocyte-derived mRNA electroporated dendritic cells (TriMixDC-MEL) plus ipilimumab in patients with pretreated advanced melanoma. J Clin Oncol. 2016;34(12):1330-1338. doi:10.1200/JCO.2015.63.4121

Newsletter
Stay up to date on recent advances in the multidisciplinary approach to cancer.
Oncology Peer Review On-The-Go: COVID-19, Cancer, and the Potential of mRNA Vaccines
March 30th 2021Mehmet Sitki Copur, MD, discussed his article in the Journal ONCOLOGY® focusing on COVID-19, messenger RNA vaccines, and the excitement surrounding its integration into the future of cancer treatment.