Targeted Therapy Options for HER2+ Breast Cancer
A Satellite Session, involving members from US Oncology, focused on treatment options for patients with HER2-positive metastatic breast cancer.
The expert panel

As part of a Satellite Sessions program, CancerNetwork hosted a panel discussion focused on the management of HER2-positive metastatic breast cancer. The program brought together experts from Texas Oncology and UT Southwestern Harold C. Simmons Comprehensive Cancer Center to discuss first-, second-, and third-line treatment options for patients with HER2-positive metastatic breast cancer and how to decide on which antibody-drug conjugate (ADC) to administer.
The panel was led by Joyce O’Shaughnessy, MD, the Celebrating Women Chair in Breast Cancer Research at Baylor University Medical Center and director of the Breast Cancer Research Program at Texas Oncology, US Oncology, in Dallas, Texas. Panel members included Heather McArthur, MD, MPH, Komen Distinguished Chair in Clinical Breast Cancer Research and associate professor of medicine in the Department of Internal Medicine at UT Southwestern Medical Center in Dallas; Isaac Chan, MD, PhD, assistant professor of medicine in the Department of Internal Medicine at UT Southwestern; Namrata Peswani, MD, assistant professor of medicine in the Department of Internal Medicine at UT Southwestern; Ashwani K. Agarwal, MD, hematology/oncology clinician at Texas Oncology; Dawn Klemow-Reed, MD, medical oncologist at UT Southwestern; and Cindy Osborne, MD, a breast medical oncologist at Texas Oncology.
ATP Binding in HER2 vs EGFR
O’Shaughnessy: We know the survival impact of these regimens. In fact, all of these have had a survival improvement in their respective phase 3 trials. Everybody knows about tucatinib [Tukysa] and that it’s a highly selective HER2 kinase inhibitor, which was interesting. I didn’t know that ATP [adenosine triphosphate] binding pockets of EGFRand HER2 differ by only 2 amino acids. Only 1 of these amino acids is involved in binding an ATP competitive inhibitor, so it’s cysteine in EGFRand serine in HER2. If you’re going to use an ATP competitive inhibitor, which tucatinib is, it competes for ATP for that binding site. In order not to inhibit EGFRs, you must be precise about which of these amino acids the tucatinib is going to bind to. It has to bind to the serine 783 [S783] on the HER2 and leave the cysteine 775 [C775] on EGFRalone.
McArthur: Is this because of the physical proximity?
O’Shaughnessy: These ATP binding “pockets” have only 2 amino acids that are different between HER2 and EGFR. Only 1 of them for each, either EGFR or HER2, is involved in getting the ATP out of the way and binding to it. They’re not necessarily right on top of each other, though. They could be across the pocket.
McArthur: It’s important that they’re not promiscuous, is that the idea?
O’Shaughnessy: Yes, because it would be easy to bind just to the pocket in general and inhibit it more globally. It’s important that tucatinib binds to the serine HER2 and leaves the cysteine EGFRalone. We’ve had lapatinib [Tykerb] and neratinib [Nerlynx] bind to both, but tucatinib is 1000 times more selective for HER2 than for EGFR. It’s known from preclinical [trial results] that it was more effective in combination with trastuzumab [Herceptin]. Some of those kinases that are inhibited by tucatinib are EGFRand HER4. There’s a bit of nonselectivity regarding kinase inhibition. The activity is restricted to HER2-related kinases vs EGFRand biochemical assays. We can’t say it’s [definitively] selected for HER2, but it’s largely selective and much better for HER2. The half-maximal inhibitory concentration [IC50] of tucatinib for HER2 is 6.9 nM. For EGFR, it’s 449 nM. For neratinib, it’s 1.8 nM. Lapatinib is not quite as potent against EGFR but still has 48 nM. Tucatinib and neratinib are similar in their IC50s, and lapatinib is much higher. The difference is that neratinib inhibits the EGFRmuch more than the tucatinib. Editors Note: Additional efficacy data conducted in this patient population can be found in TABLE.1-3
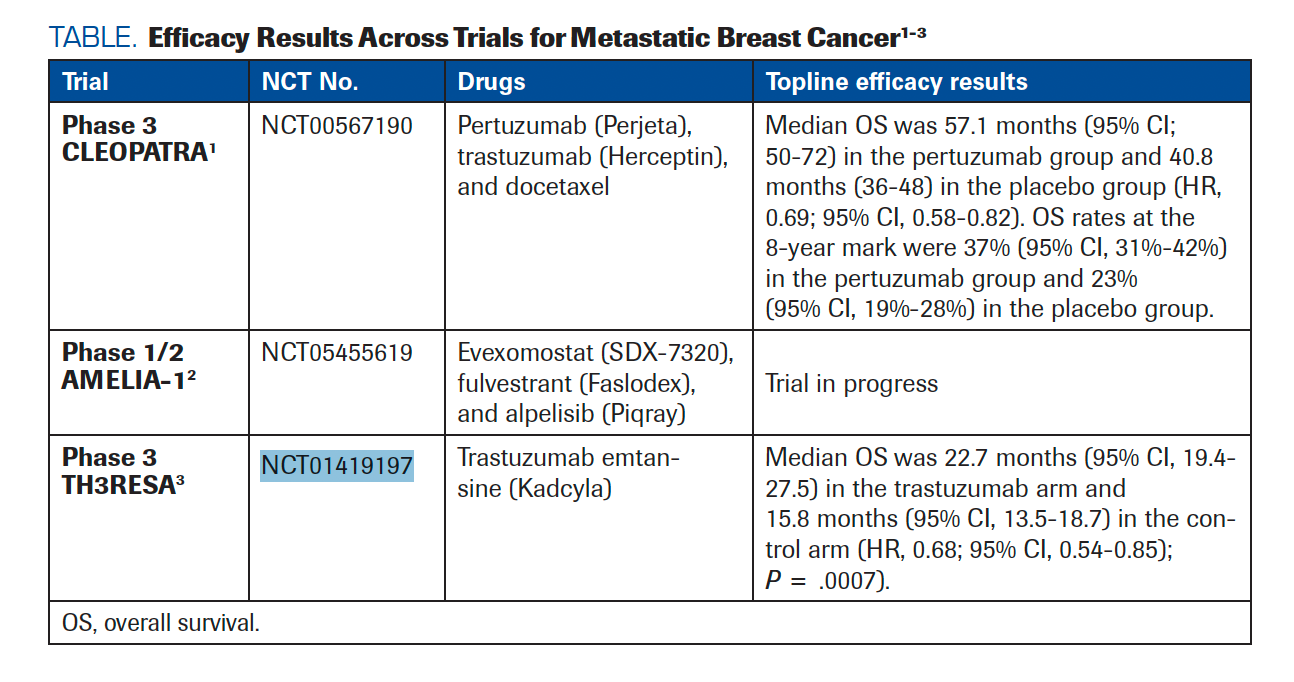
TABLE. Efficacy Results Across Trials for Metastatic Breast Cancer1-3
Heterogeneity in HER2-Positive Tumors
Chan: Do we know anything about the effectiveness of neratinib vs tucatinib in a HER2 heterogeneous tumor?
McArthur: How can we evaluate that? What exactly is the nature of the heterogeneity of HER2-positive tumors?
O’Shaughnessy: This is discussed in the phase 2 HER2CLIMB study [NCT02614794].4 I remember when we were trying to enroll in this trial that it was a heavily pretreated group. [These were patients with] prior pertuzumab [Perjeta], trastuzumab, and trastuzumab emtansine [T-DM1; Kadcyla]. This was the study where [all the participants] had to have a brain MRI. The researchers found that 48% of the patients had brain metastases, and half of those were active. They hadn’t been treated. They were found on the MRI, but they weren’t clinically known about. The other half knew they had brain metastases and had been treated. Still, they were allowed in the study, even if they had received stereotactic radiosurgery to a few of [the metastases]. If some of the other [metastases] started to grow, if they were not symptomatic, [patients] were allowed in the study. It was a new way of letting [patients with] untreated brain metastases in the study. They were [randomly assigned] 2:1 to the tucatinib/trastuzumab/capecitabine arm vs placebo/trastuzumab/capecitabine. The primary end point was progression-free survival [PFS], and secondary end points were PFS in patients with brain metastases, overall survival [OS] in all patients, response rate, and safety. The women [in the study] were around 54 to
55 years old, so [relatively] young. A total of 46% and 48% [in each respective arm] had a presence or history of brain metastases. In the metastatic setting, they had received a median of 3 prior regimens. The PFS was [5.4 months] vs 7.6 months in the placebo and treatment arms, respectively. There is a statistical significance there. The HR for disease progression or death was 0.54 [95% CI, 0.42-0.71; P < .001]. The estimated PFS at 1 year was 33.1% in the tucatinib group and 12.3% in the placebo group.
I was surprised when I saw these data for the first time. I was surprised at the survival improvement [between] 3 drugs vs 2 drugs. We hadn’t seen that with neratinib. We had seen no survival data in that trial.5 Here we had a 5.5-month improvement in OS [HR, 0.66; 95% CI, 0.50-0.88; P = .005], and the OS at 2 years was 44.9% in the tucatinib combination group and 26.6% in the placebo combination group. That was significant. What are your thoughts about these data?
Agarwal: These are amazing data, [especially because] this is in patients who are heavily pretreated. However, if the patient has a brain mass that I identify, I go to use tucatinib up front because I think there is some penetration. After the first line, I don’t go to T-DM1. I would rather go straight to tucatinib if they have brain metastases.
O’Shaughnessy: That’s for sure because the data in phase 3 trials for T-DM1 and brain metastases don’t exist.
Agarwal: These are very powerful data. After so many lines of treatment, you’re giving another 2 years of survival to these patients. They’ve already lived another 10 years before or 20 years before, and you’re extending by another 2 years.
McArthur: It was brave to enroll patients with active brain metastases, so to see this kind of reward in that high-risk population was unprecedented and gratifying.
O’Shaughnessy: We know that 82% of patients on the tucatinib/trastuzumab/capecitabine regimen had diarrhea, and 13% [had] grade 3. There was a bit more hand-foot syndrome and a bit more nausea [58.4% vs 43.7%] with tucatinib than without. Fatigue was the same [in both arms]. There was a bit more vomiting [35.9% vs 25.4%] and more stomatitis [25.5% vs 14.2%]. It looked like the addition of this agent was exacerbating the capecitabine toxicities a bit. [There were also] elevations in aspartate aminotransferase and alanine aminotransferase [21.3% and 20.0% vs 11.2% and 6.6%, respectively,] and elevations in bilirubin [18.6% vs 10.2%]. There was a differential here in toxicity; however, a lot of us are mitigating that by [the way we administer treatment]. Do you start with the recommended dose of treatment?
Osborne: I usually do.
Third-Line Treatment Options
O’Shaughnessy: How do you decide what to do for third-line treatments? Typically, you use the phase 3 CLEOPATRA study [NCT00567190]1 treatment of pertuzumab, trastuzumab, and docetaxel for the first line.6 You use trastuzumab deruxtecan [T-DXd; Enhertu] for the second line.How do you decide what to do for the third line?
Agarwal: I tend to go toward tucatinib because most of these patients, no matter what, will develop brain metastases. With tucatinib, there may be a protective effect. It postpones or prevents it.
O’Shaughnessy: Are you scanning all your patients’ brains?
Agarwal: Yes, I am.
O’Shaughnessy: Then you certainly would prioritize tucatinib.
Agarwal: Yes, because even if they are in their third line of treatment, they are still at risk for developing brain metastases.
Peswani: I’ve had a lot of patients who had toxicity with the tucatinib/capecitabine combination, so I tend to go to T-DM1 and then use tucatinib/capecitabine after that mainly to try to preserve patients’ quality of life and performance status. I’ve just found tucatinib/capecitabine to be a harder regimen for the patients to tolerate.
O’Shaughnessy: What’s been your impression of the efficacy of T-DM1 after T-DXd?
Peswani: Unfortunately, I had a patient who received T-DM1 after T-DXd who passed away less than 4 weeks ago. She developed pneumonitis, ended up in the hospital, went to an inpatient hospice, and passed away. She was the only one who had received T-DXd followed
by T-DM1.
O’Shaughnessy: The pneumonitis was an adverse effect [AE] of the T-DM1, not the T-DXd?
Peswani: Yes.
O’Shaughnessy: T-DM1 does have the risk of pneumonitis as well, though much less.
Agarwal: The toxicity comes when you start with capecitabine treatment at 750 mg/m2. That’s what you have
to manage.
Klemow-Reed: I typically will use tucatinib because I think it’s a more powerful combination. The only time I wouldn’t is if the patient’s performance status would not allow it.
O’Shaughnessy: So you start with the full dose of tucatinib. With capecitabine, you start with 800 mg/m2, so lower [than the standard of care], and adjust the treatment amount depending on how patients feel.
Klemow-Reed: Yes.
Chan: I agree with Dr Peswani. A lot of the time, patients are shy with the capecitabine. I tend to base treatment on patient preference. I had a patient who had T-DXd–developed pneumonitis. We then switched her to T-DM1, and she continued to have pneumonitis. Then we just did a very prolonged [taper of steroids].
Peswani: My patient had COVID-19 while [taking] T-DXd, so there were multiple reasons to have worsening pneumonitis.
O’Shaughnessy: Do you think that T-DM1 had any effect on the prolonged pneumonitis, or do you think it was all due to the T-DXd?
Chan: I think it was due to the T-DXd. I’ve had cases where patients have gone to the intensive care unit and then [died] from the pneumonitis related to the treatment, so it’s severe.
McArthur: I tend to use T-DXd or the HER2CLIMB regimen earlier on in the course of the disease. I use the better [treatments] early on to get the maximum benefit. I might use HER2CLIMB as a second-line treatment before T-DXd, depending on the patient’s preference, because it is so much easier to administer than T-DXd. I have a patient who was on T-DXd for 6 years with no AEs or no abnormalities detected. Then I had a patient who had 2 doses with maximum prophylaxis who said, “I don’t care if this is the best drug on the planet; I never want to see this drug ever again.” I’ve seen the range of experiences with T-DXd. I find the HER2CLIMB regimen to be very manageable. I dose capecitabine on that regimen like I do in my practice otherwise, which is “spidey sense” dosing, so looking at how strong they are, how big they are. I might start with a flat dose for an average-sized person at 1500 mg/m2 and 1000 mg/m2 and then titrate up or down from there. I don’t adhere to the regimen prescription. I extrapolate from what I do in my practice otherwise, but I give the full dose of tucatinib and trastuzumab.
References
- Swain SM, Miles D, Kim SB, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21(4):519-530. doi:10.1016/s1470-2045(19)30863-0
- Cornelius P, Salomon N, Browning D, et al. The Amelia-1 study: A phase 1b/2 trial of evexomostat (SDX-7320) plus fulvestrant and alpelisib in patients with advanced breast cancer at risk for alpelisib-induced hyperglycemia. J Clin Oncol. 2023;41(16_suppl):TPS1129-TPS1129. doi:10.1200/jco.2023.41.16_suppl.tps1129
- Krop IE, Kim SB, Martin AG, et al. Trastuzumab emtansine versus treatment of physician’s choice in patients with previously treated HER2-positive metastatic breast cancer (TH3RESA): final overall survival results from a randomised open-label phase 3 trial. Lancet Oncol. 2017;18(6):743-754. doi:10.1016/s1470-2045(17)30313-3
- Murthy RK, Loi S, Okines A, et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N Engl J Med. 2020;382(7):597-609. doi:10.1056/nejmoa1914609
- FDA approves neratinib for metastatic HER2-positive breast cancer. FDA. Updated February 26, 2020. Accessed December 11, 2023. https://tinyurl.com/mrdys53w