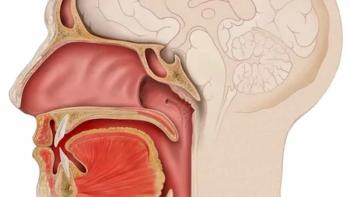
In October 2023, the FDA approved frontline toripalimab-tpzi plus cisplatin and gemcitabine for adult patients with metastatic or locally advanced nasopharyngeal carcinoma, as well as single-agent toripalimab for recurrent, unresectable, or metastatic disease that has progressed on or following prior chemotherapy.