
Guang Peng, MD, PhD spoke to Cancer Network about the recent discovery that mTORC1 can help determine cell fate after DNA damage.

Your AI-Trained Oncology Knowledge Connection!


Guang Peng, MD, PhD spoke to Cancer Network about the recent discovery that mTORC1 can help determine cell fate after DNA damage.

Dr. Raghu Kalluri speaks with Cancer Network about the role of exosomes in cancer progression ahead of his presentation at ESMO 2018.

In his latest blog, Craig Hildreth explores the terror of the black box warning and how the fear of toxicities affects his patients-and his staff.

Dr. Ian Flinn spoke with Cancer Network about incorporating the newest approved therapies for the management of CLL.

In this article, we describe the mechanisms via which interactions between herbs and prescription drugs may occur, and highlight four popular herbs and a medicinal mushroom commonly used by cancer patients, along with reports of their interactions with standard drugs.
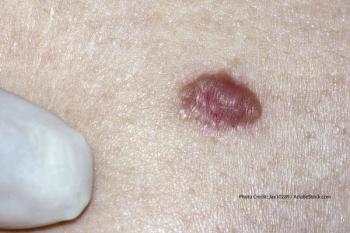
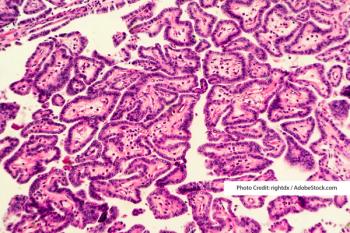
A new study in Cancer Management and Research found a correlation between the age of patients with papillary thyroid cancer and lymph node positivity.
LOXO-292, which selectively targets RET and has demonstrated preclinical activity against activating RET fusions/mutations, holds promise.

We take a look at how a healthy diet can improve quality of life in breast cancer survivors.
Cemiplimab (Libtayo), approved for advanced cutaneous squamous cell carcinoma, is intended for those not eligible for curative surgery or radiation.

Cervical cancer is the most common HPV-associated cancer. What are some of the others? Take our quiz to test your knowledge.

Dianne Shumay, PhD, director of psycho-oncology at UCSF, weighs in on how we can use psychological interventions to improve stress levels in cancer patients.
A study shows gene variants of uncertain significance are frequently reclassified, necessitating amended test result reports.

The FDA authorized the first next-generation sequencing test to detect minimal residual disease in blood cancers.

Patients with von Hippel-Lindau disease may have a new treatment option in the antiangiogenic therapy pazopanib.

Nanotechnological strategies are making preclinical strides toward more effective, less toxic, immunotherapies.

A study shows TRK gene fusions in hematologic cancers may respond to larotrectinib.
The eighth edition outperforms the seventh edition in predicting survival differences in patients with papillary thyroid cancer and follicular thyroid cancer.
The study confirmed a significant effect of male sex on PTC-specific patient survival in individuals with the most frequent oncogenic mutation.

Professor Judi Fouladbakhsh speaks with Cancer Network about evidence-based holistic approaches to cancer care.
Certain malignancies may possess a common and targetable matrix response, which affects disease pathogeneis, according to a study in Cancer Discovery.

Dr. Dmitriy Zamarin speaks with Cancer Network about the findings of a phase II trial in platinum-resistant ovarian cancer patients.
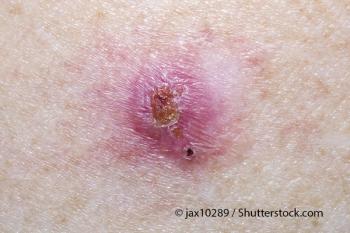
Individuals who develop frequent basal cell carcinomas may have an increased prevalence of germline mutations in DNA repair genes and an increased malignancy risk.
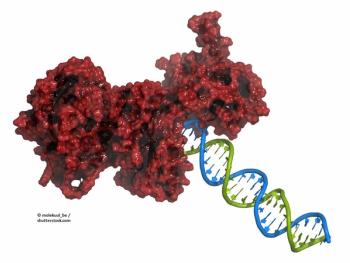
New research examines the mechanisms underlying why most patients with high-grade serous ovarian cancer develop resistance to PARP inhibitors.

Linda Jacobs, PhD, CRNP, director of the Cancer Survivorship Center, comments on the incidence of erectile dysfunction in childhood cancer survivors.

Researchers have developed a mouse model of high-grade serous carcinoma that could prove useful in the preclinical testing of prevention strategies for ovarian cancer.
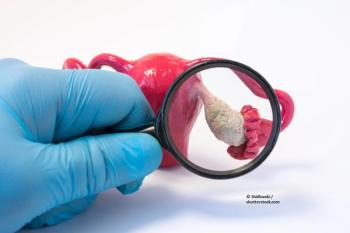
Optical coherence tomography and multispectral fluorescence imaging have demonstrated promising results in the early detection of ovarian cancer.

Breast and gynecologic cancer patients undergoing chemotherapy may experience improvement in cancer-related fatigue with integrative medicine approaches.

Dr. Ranjit Manchanda speaks with Cancer Network about using population testing to predict and prevent gynecologic cancers.
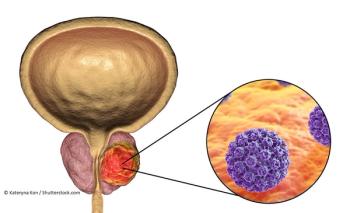
A particular gatekeeper-the nuclear pore protein called POM121-traffics molecules that increase tumor aggressiveness in prostate cancer.