45 Application of the 7-Gene Biosignature in Palpable Versus Nonpalpable Ductal Carcinoma In Situ in a Black Patient Population: Does Palpability Suggest a More Aggressive Genomic Risk?
Background
Palpable ductal carcinoma in situ (p-DCIS) is a rare entity—only 10% of patients present with a clinical mass. We have noticed high rates of palpability among our African American patient population.
Previous studies have suggested that p-DCIS may be associated with more aggressive clinicohistologic features such as higher nuclear grade, comedonecrosis, HER2-neu positivity, and being hormone receptor-negative. DCISionRT (DRT) is a 7-gene biosignature that predicts recurrence risk, defined as either a low (0-3) or elevated (3.1-10) score. We hypothesized that p-DCIS cases in our African American patient population would have higher DRT scores when compared with nonpalpable cases (n-DCIS).
Methods
An institutional review board (IRB)-approved retrospective chart review was performed on all cases of DCIS identified at a single institution from 2021 to 2023. All cases had the results of the DRT scores. We analyzed clinical, histologic, and demographic features and used descriptive statistics to compare groups.
DRT Scores
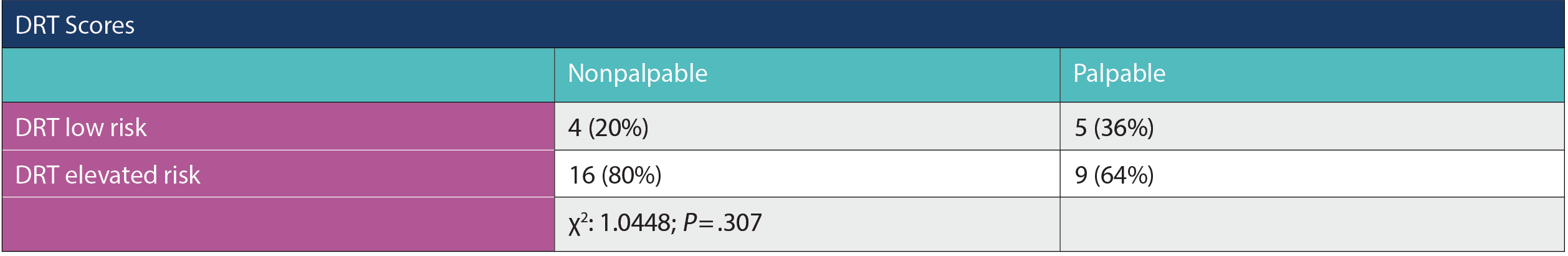
Results
Thirty-four patients were identified in the cohort and all self-identified as African American. Fourteen patients (41%) presented with a clinically palpable mass, while 20 (59%) were detected on imaging. Overall, 25/34 (74%) had an elevated DRT score. Nine of the 14 patients (64%) of p-DCIS cases had an elevated DRT score while 5 (36%) had low scores. Out of n-DCIS cases, 16 (80%) had elevated DRT scores while 4 (20%) had low scores (Pearson chi: 1.0448; P = .307).
Conclusion
While invasive breast cancer is known to be more aggressive among African American women, studies have failed to identify a more aggressive in situ histology in this population. Here we report a higher-than-average rate of palpability at 41%. While most of the patients in our cohort had an elevated DRT score overall, there was no significant difference when comparing both groups. Further studies are warranted to identify factors that may predict in situ or invasive recurrence in this population.
