A 64-Year-Old Man With Germline BRCA2-Mutated Breast Cancer: Known and Unknown Aspects of Male Breast Cancer
This study presents a male breast cancer case with a germline BRCA2 mutation and discusses the epidemiologic, pathologic, and clinical characteristics along with treatment and follow-up recommendations in view of our recent understanding of the disease.
Copur is a medical oncologist/hematologist and medical director of oncology at Morrison Cancer Center, Mary Lanning Healthcare in Hastings, Nebraska, and is a professor at the University of Nebraska Medical Center adjunct faculty. He is also an Editorial Advisory Board member at ONCOLOGY®.

Kelly is a radiation oncologist at the Morrison Cancer Center.

Lintel is a pathologist at Mary Lanning Healthcare.

Riley is a genetic counselor at Mary Lanning Healthcare.

Herold is a radiologist at Morrison Cancer Center, Mary Lanning Healthcare.

Faris is a surgeon at Mary Lanning Healthcare.

Wedel is a pathologist at Mary Lanning Healthcare.

Horn is a pathologist at Mary Lanning Healthcare.

Abstract
Male breast cancer is a rather uncommon and understudied disease. It accounts for less than 1% of all breast cancers, but in recent decades its frequency has been on the rise. Clinical trials of breast cancer have traditionally excluded men. Due to the lack of large-scale prospective studies, most published data come from single-institution, small-cohort studies, and treatment recommendations are based on the extrapolation of data from clinical trials enrolling only women. Although to some extent etiology, diagnosis, and treatment characteristics can be similar, male breast cancer exhibits some distinct features. Men tend to be diagnosed with breast cancer at an older age and at a more advanced stage. A better understanding of the biologic features, clinically relevant differences, effective treatments, and outcomes of male breast cancer is crucial to appropriately manage these patients. We present a male breast cancer case with a germline BRCA2 mutation and discuss the epidemiologic, pathologic, and clinical characteristics along with treatment and follow-up recommendations in view of our recent understanding of this disease.
Oncology (Williston Park). 2021;35(8):480-484.
DOI: 10.46883/ONC.2021.3508.0480
Introduction
Male breast cancer is a rare disease accounting for less than 1% of all breast cancers, and less than 1% of all cancers in men. Worldwide estimated incidence of male breast cancer has been reported to be 1 per 100,000 men per year, with a mean age at diagnosis of 60 to 70 years.1,2 The average age at diagnosis is approximately 5 years older than it is for women (67 years vs 62 years, respectively).3 Based on data from the Surveillance, Epidemiology, and End Results (SEER) program, the age-adjusted incidence rate has increased from 0.85 cases per 100,000 in 1975 to 1.43 cases per 100,000 in 2011. Black men appear to be at greater risk than non-Hispanic White men.4,5
Male breast cancer seems to be an estrogen-driven disease; it has been likened to late-onset, postmenopausal, estrogen receptor/progesterone receptor–positive (ER+/PR+) female breast cancer.6 Male breast cancer tends to occur at a later age and to present at a more advanced stage, with more hormone receptor positivity.7 However, there are differences, resulting in an ongoing debate regarding the level of similarity.
Male breast cancer may be a unique tumor type with biological and clinicopathological features distinct from female breast cancer. However, similar to female breast cancer, family history has been shown to be an important risk factor.8,9 The risk of breast cancer doubles for men who have a first-degree relative with the disease.10 Population-based studies indicate that mutations in the 2 major high-penetrance breast cancer genes, BRCA1 and BRCA2, account for approximately 10% of male breast cancers11; 0% to 4% of men with breast cancer have BRCA1 mutations and 4% to 16% have BRCA2 mutations.8,9,12,13 The lifetime risk of developing male breast cancer has been estimated to be in the range of 1% to 5% for BRCA1 and 5% to 10 % for BRCA2 mutation carriers, compared with a risk of 0.1% in the general population.14-17
Genes other than BRCA1/2 may also be involved. A truncating mutation (CHEK2*1100delC) has been shown to increase the risk of male breast cancer by a factor of 10, compared with the risk among men who do not have the mutation.13,18 Also reported have been mutations in other genes, including the PTEN tumor suppressor gene and the androgen receptor gene, as well as TP53 mutations (Li-Fraumeni syndrome), PALB2 mutations, and mismatch repair mutations associated with hereditary nonpolyposis colorectal cancer (Lynch syndrome).13,19-21 Other risk factors for male breast cancer are hormonal imbalances (caused by liver disease, Klinefelter syndrome, or obesity) and environmental factors (eg, chronic exposure to heat or radiation).22-26
Case
A White man, aged 64 years, presented with complaints of a palpable lump on the upper outer quadrant of his left breast along with some pain for the past several months. On physical exam, he was noted to have a 2.5-cm left breast mass along with retraction of the retroareolar and nipple region without discharge (Figure 1). A left breast mammogram showed scattered areas of fibroglandular density throughout the breast. Ultrasound (US) confirmed a 1.9 cm × 1.7 cm irregular hypoechoic mass with no identifiable axillary nodes. US-guided biopsy reported an invasive ductal carcinoma grade 3, ER+ (85%), PR negative (<1%), HER2 negative, and Ki-67 (30%). MRI of the bilateral breasts confirmed a retroareolar mass on the left consistent with known malignancy, with no chest wall or axillary involvement, and normal appearance of the right breast (Figure 2).
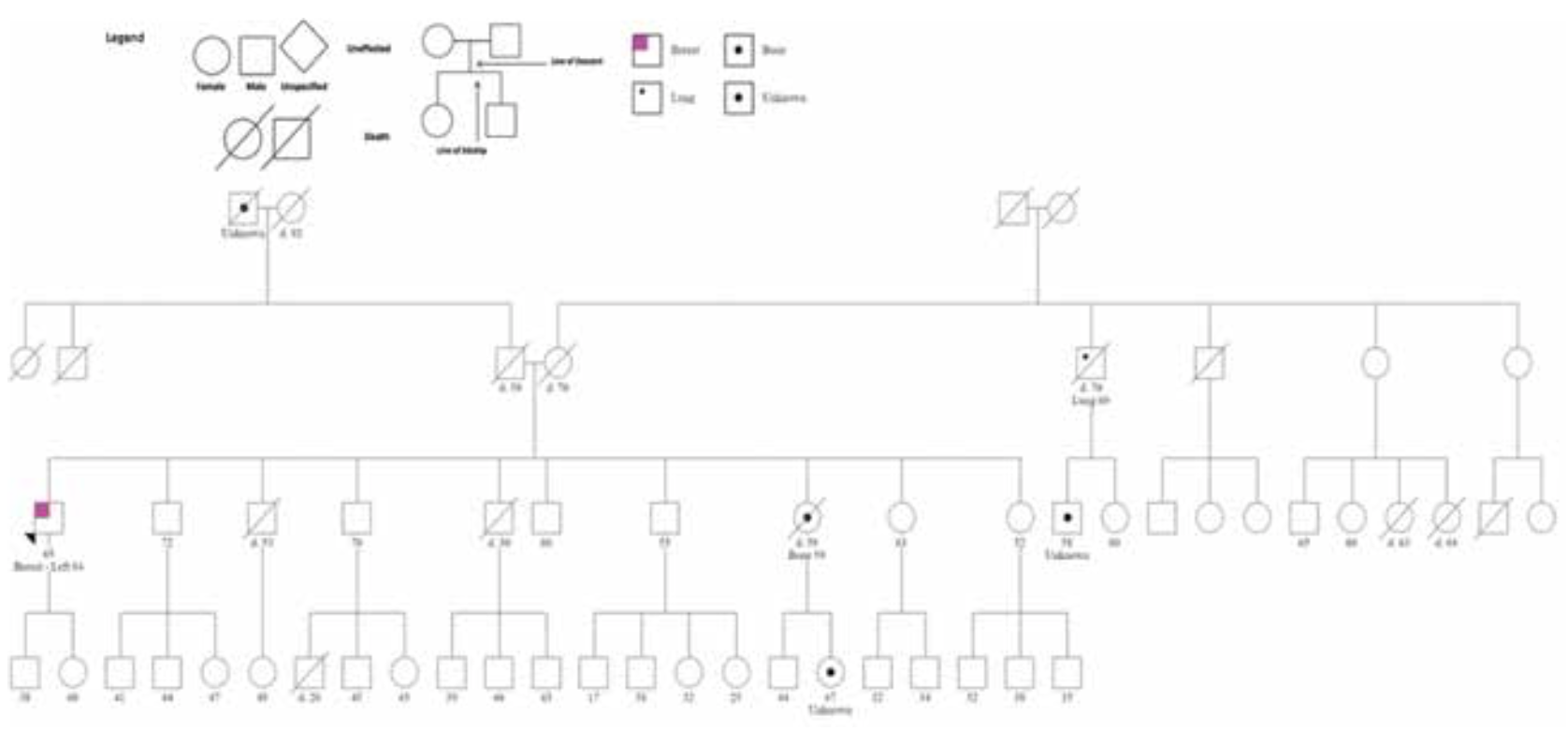
The patient was treated with left mastectomy and axillary sentinel lymph node (LN) sampling. Pathology revealed a 2.1-cm invasive ductal carcinoma, grade 3, with no ductal carcinoma in situ (DCIS) (Figure 3). One of the 3 sentinel nodes showed isolated tumor cells. Pathologic stage was pT2, pN0(i+). Tumor Oncotype-Dx Breast Recurrence Score (BRS) was 42. Pretest cancer genetic counseling followed by germline targeted multigene mutation analysis revealed a pathogenic mutation, c.1147delA in the BRCA2 gene, consistent with hereditary breast and ovarian cancer (HBOC) syndrome. Family pedigree is shown in Figure 4.
FIGURE 1. Left breast mass with retraction of retro areolar and nipple region without discharge.
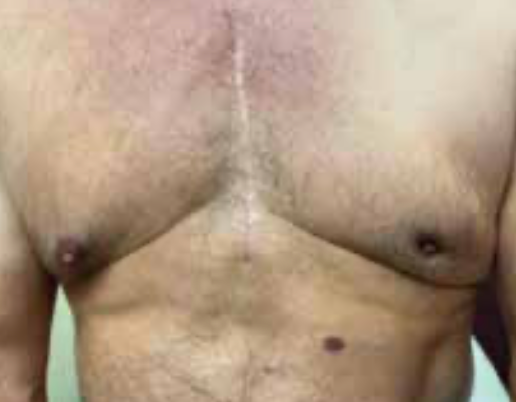
FIGURE 2. Retro areolar mass consistent with known malignancy with no chest wall or axillary involvement on the left and normal appearance of the right breast.
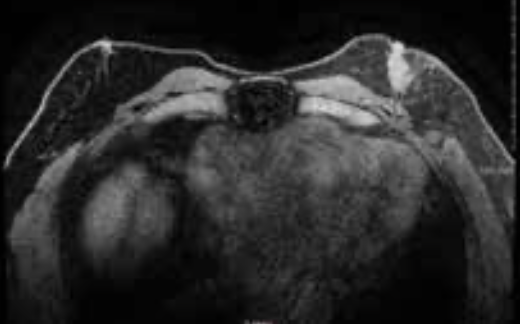
FIGURE 3. 40x H&E, scattered mitotic figures with marked pleomorphic cells lacking gland formation constituting grade 3 ductal carcinoma.
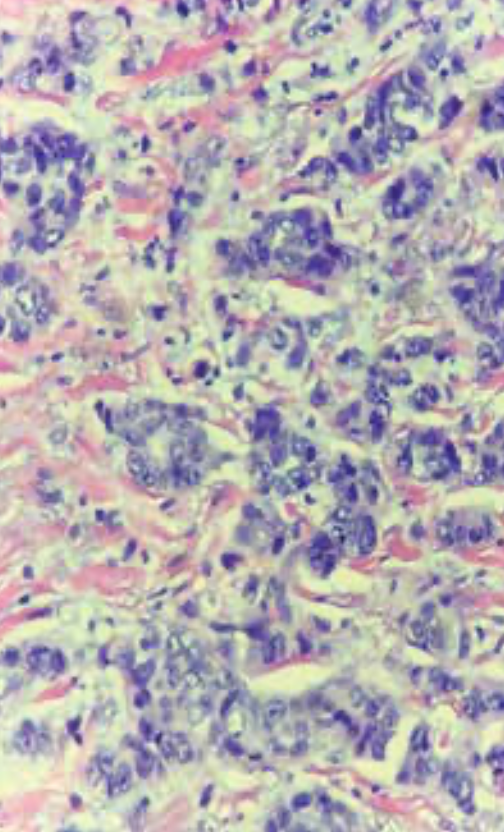
FIGURE 4. Family Pedigree
Clinical and Pathological Characteristics
The most common clinical presentation in men with breast cancer is a painless, retroareolar mass. Nipple retraction, bleeding from the nipple, skin ulceration, and palpable axillary adenopathy are among other reported signs and symptoms.27 To differentiate from gynecomastia, a highly prevalent condition, breast imaging should be considered. The American College of Radiology recommends initial US for men aged younger than 25 years with an indeterminate palpable mass. For men 25 years or older or those with questionable findings on physical examination, mammography is recommended as the initial diagnostic test followed by US if the mammographic findings are inconclusive or suggestive of cancer.28 On mammograms, male breast cancers appear as eccentric, retroareolar masses with irregular, spiculated edges.29,30 Due to low public awareness and the lack of screening mammograms for men, male breast cancers are more frequently diagnosed as larger tumors with regional nodal metastases.31 Staging for breast cancer in men is the same as for women with no gender-specific recommendations.
Men with abnormal imaging findings suggestive of malignancy should undergo core biopsy to confirm the diagnosis. Approximately 10% of men with breast cancer present with DCIS compared with 20% to 25% of women. Lobular carcinoma in situ in men is very rare due to anatomical absence of terminal lobules in the male breast. Most male breast cancer cases are invasive ductal carcinomas, but rarely papillary cancers (in 2% to 3% of cases) and mucinous cancers (in 1% to 2% of cases) can be seen.3,32,33 Invasive lobular carcinoma is much less prevalent among men, accounting for only 1% to 2% of cases.3,34 In the largest series of male patients with breast cancer analysis, tumor samples from 1483 men showed 99% ER+, 97% androgen receptor positive, 87% PR+, and 9% HER2+. Less than 1% of male breast cancer specimens were triple negative.34
In a genomic profiling study of 59 male patients with breast cancer, 29% of samples were classified as luminal A–like and 71% as luminal B–like, with a heterogeneous repertoire of somatic genetic alterations. PIK3CA and TP53 mutations, and losses of 16q, which are typically seen in ER+/HER2-negative female breast cancers, were less frequent in male breast cancer samples. Mutations affecting DNA repair–related genes were more frequent.35 Using unsupervised clustering approach, a gene-expression analysis study identified 2 unique and stable subgroups of male breast cancer with differences in tumor biological features and outcome: luminal M1 tumors with low expression of ER signaling genes, aggressive clinical behavior, and worse prognosis; and luminal M2 tumors with activated ER signaling genes, upregulated immune response genes, and favorable clinical behavior. Both subgroups differed from the established 4 intrinsic subgroups of female breast cancer.36
Treatment
Evidence from observational studies shows that breast-conserving therapy in men is associated with similar survival rates as mastectomy.37-40 Interestingly, however, men with breast cancer, even in early-stage disease, are being treated with mastectomy and either with axillary LN dissection or sentinel node biopsy despite the absence of any medical contraindication for breast conservation. Based on a SEER registry data analysis, only 18% of men with T1N0 tumors underwent breast-conserving surgery.38 Sentinel node biopsy, the standard approach for women with a clinically negative axilla, has been shown to be feasible and effective in men with breast cancer.41,42
Due to the rarity of male breast cancer and lack of randomized trials, the role of radiotherapy after mastectomy in node-positive male breast cancer has not been well studied. Hypofractionated radiotherapy regimens have not been tested in male breast cancer, as men were excluded from the UK START trials as well as the Canadian trial(NCT00156052.)43-44As adjuvant radiotherapy has been shown to decrease the rate of relapse, adjuvant radiotherapy should be offered to men who have undergone breast-conserving surgery, based on the same guidelines developed for women with breast cancer.2 Indeed, population-based observational studies suggest a benefit in this setting.45-47
In real-world practice, however, radiotherapy seems to be underutilized in men with breast cancer. Also, patient compliance rates with adjuvant radiation therapy seem to be generally low. Based on the SEER data, only 42% of men with stage I breast cancer received radiotherapy after breast-conserving surgery.38,48 Recently published National Cancer Data Base study data are somewhat encouraging and show that adjuvant radiotherapy was given to 70% of patients undergoing breast-conserving surgery in stage I to III male breast cancer.49 Worldwide data involving 1483 male patients with breast cancer treated between 1990 and 2010, examined in a retrospective study in collaboration with the European Organization for Research and Treatment of Cancer, the Translational Breast Cancer Research Consortium, the North American Breast Cancer Group, and the Breast International Group period, showed that about one-half of men who were treated with breast-conserving surgery did not receive radiotherapy.34
Due to exclusion of male patients with breast cancer from clinical trials involving female breast cancer, there are no randomized studies of adjuvant or neoadjuvant chemotherapy or of HER2-targeted therapy in this population. There has been 1 reported prospective National Cancer Institute study evaluating adjuvant chemotherapy in 31 men who had stage II breast cancer with LN involvement. Patients were treated with mastectomy and 12 cycles of cyclophosphamide, methotrexate, and fluorouracil between 1974 and 1988. This cohort was followed for more than 20 years; survival was 80% at 5 years, 65% at 10 years, and 42% at 20 years, comparing favorably with historical rates.50 In a more recent study of 32 men with breast cancer treated with systemic adjuvant/neoadjuvant chemotherapy, the 5-year and 10-year overall survival rates were 86% and 75%, respectively, for men with LN-negative disease, and 70% and 43%, respectively, for men with LN+ disease. For men with LN+ disease, adjuvant chemotherapy was associated with a lower risk of death (HR, 0.78).51 Additional observational cohort studies in men have suggested improved survival with the use of adjuvant chemotherapy.52-55
Since the majority of male breast cancers are HR+, endocrine therapy plays an important role in both in the adjuvant and metastatic settings. Observational studies of adjuvant treatment with tamoxifen suggest a survival benefit. Male patients with breast cancer were shown to benefit more from endocrine therapy with tamoxifen than from aromatase inhibitors (AIs) in a cohort of 257 patients.56,57 Population-based series have shown inferior survival rates when men were treated with adjuvant AIs as compared with tamoxifen. The lack of complete estradiol suppression due to feedback loops in the hypothalamus and pituitary glands has been shown to be the reason for the lower efficacy of AIs in men.58,59 For men who are not good candidates for tamoxifen, a GnRH analogue with or without an AI can be used to overcome this.60
Gene expression profiling assays have been widely used to quantify the risk of recurrence as well as to guide systemic therapy in early-stage breast cancer in women. The 21-gene BRS and 70-gene MammaPrint assay tests provide individualized estimates of distant recurrence risk and help predict benefit from adjuvant chemotherapy in women with ER+ breast cancer.61 Data on the utility of these assays in male patients with breast cancer are limited. Crozier et al reported that while similar MammaPrint index distributions between male and female breast cancer tumors existed, there were distinct biological pathways between male and female breast cancer—notably, upregulation of estrogen response and MTORC1 signaling in male compared with female breast cancer specimens.62 Similarly, Oncotype DX results have shown comparable mean BRS in men and women, but mean quantitative gene expression for genes related to ER, proliferation, and invasion were higher in men compared with women.63-65
Genetic Counseling and Management
Germline mutations in BRCA1/2 are inherited in an autosomal dominant manner with the majority of individuals having inherited it from a parent (de novo rate ≤5%).66 The parent with the mutation may or may not have had a cancer diagnosis for several reasons, including penetrance of the mutation, gender and age of the parent, cancer risk reduction due to screening/prophylactic surgeries, or early death.67 It is appropriate to offer germline testing to both parents of an individual with a BRCA1/2 mutation to determine which side of the family is at risk; however, the parent with the family history of BRCA1/2-related cancers can be tested first. Being a carrier of a BRCA1/2 mutation also comes with reproductive risks. For example, in cases where both partners carry a BRCA2 mutation, there may be a high risk for the offspring to develop Fanconi anemia, a rare autosomal recessive condition.68 Additionally, a case has been found in which biallelic BRCA1 mutations caused a Fanconi anemia–like disorder.69 The current estimates of the frequency of BRCA1 and BRCA2 gene mutations in the population is approximately 1 in 500 individuals.70
Male carriers of BRCA1/2 mutations are at increased risk for development of several types of cancers, including breast (BRCA1, 1.2% lifetime; BRCA2, 7% to 8% lifetime), prostate (BRCA1, exact risk unknown; BRCA2, 20% to 30% lifetime), pancreas (BRCA1, 2% to 3% lifetime; BRCA2, 3% to 5% lifetime), and melanoma (BRCA2, 3% to 5% lifetime).14,16,71-73 The National Comprehensive Cancer Network (v1.2021) recommends breast self-exam training and education starting at age 35 years, a clinical breast exam every 12 months starting at age 35 years, and consideration of annual mammogram screening in men with gynecomastia starting at age 50 years or 10 years before the earliest known male breast cancer in the family (whichever comes first).74
While tamoxifen can be used as a chemopreventive agent for female breast cancer, there are no data for men in this setting. Similarly, prophylactic mastectomy has not been offered due to lack of data to support this type of risk reduction. However, Jemal et al.75 reported an increase in contralateral mastectomy in men diagnosed with a unilateral breast cancer. The procedure was more common in younger, White, and privately insured patients. For male patients harboring BRCA2 mutations,prostate cancer surveillance with annual prostate-specific antigen and digital rectal examination beginning at age 40 years is recommended, and these should be considered for male BRCA1 carriers as well. Pancreatic cancer screening with contrast-enhanced MRI/ magnetic resonance cholangiopancreatography and/or endoscopic US is recommended for BRCA1/2 carriers at age 50 years (or 10 years younger than the earliest pancreatic cancer in the family, whichever comes first who have 1 or more first- or second-degree relatives with pancreatic cancer. No BRCA1/2-specific screening guidelines exist for melanoma, but general melanoma management, with annual full-body skin examination and minimizing ultraviolet exposure, is a sensible option.74
Conclusions
Here, we discuss a male patient with breast cancer presenting with a palpable mass; surgical treatment and staging led to a diagnosis of pT2, pN0(i+) invasive ductal carcinoma of the breast with a Oncotype Dx BRS of 42. Pretest cancer genetic counseling followed by germline targeted multigene mutation analysis revealed a pathogenic mutation, c.1147delA in the BRCA2 gene, consistent with HBOC syndrome.
Although breast cancer in men and in women is similar in some ways, there
remain gaps in our understanding of breast cancer in men. Examination of the biology of the disease would help identify the differences and determine whether identified differences have therapeutic implications. Further work that focuses on appropriate treatment of men with breast cancer is needed.
Outcome of the Case
Following surgical treatment, the patient was treated with adjuvant chemotherapy (docetaxel/ cyclophosphamide) for 4 cycles; postmastectomy radiation; and adjuvant tamoxifen therapy to be given for 5 years. Currently he does not have any evidence of disease.
Financial Disclosure: The authors have no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
References
- Ly D, Forman D, Ferlay J, Brinton LA, Cook MB. An international comparison of male and female breast cancer incidence rates. Int J Cancer. 2013;132(8):1918-1926. doi:10.1002/ijc.27841
- Korde LA, Zujewski JA, Kamin L, et al. Multidisciplinary meeting on male breast cancer: summary and research recommendations. J Clin Oncol. 2010;28(12):2114-2122. doi:10.1200/JCO.2009.25.5729
- Giordano SH, Cohen DS, Buzdar AU, Perkins G, Hortobagyi GN. Breast carcinoma in men: a population-based study. Cancer. 2004;101(1):51-57. doi:10.1002/cncr.20312
- SEER Cancer Statistics Review (CSR) 1975-2014. National Cancer Institute/Surveillance, Epidemiology, and End Results Program. Updated April 2, 2018. Accessed July 28,2021. https://seer.cancer.gov/archive/csr/1975_2014/
- O’Malley C, Shema S, White E, Glaser S. Incidence of male breast cancer in California, 1988-2000: racial/ethnic variation in 1759 men. Breast Cancer Res Treat. 2005;93(2):145-150. doi:10.1007/s10549-005-4517-z
- Brinton LA, Key TJ, Kolonel LN, et al. Prediagnostic sex steroid hormones in relation to male breast cancer risk. J Clin Oncol. 2015;33(18):2041-2050. doi:10.1200/JCO.2014.59.1602
- Anderson WF, Jatoi I, Tse J, Rosenberg PS. Male breast cancer: a population-based comparison with female breast cancer. J Clin Oncol. 2010;28(2):232-239. doi:10.1200/JCO.2009.23.8162
- Basham VM, Lipscombe JM, Ward JM, et al. BRCA1 and BRCA2 mutations in a population-based study of male breast cancer. Breast Cancer Res. 2002;4(1):R2. doi:10.1186/bcr419
- Ottini L, Masala G, D’Amico C, et al. BRCA1 and BRCA2 mutation status and tumor characteristics in male breast cancer: a population-based study in Italy. Cancer Res. 2003;63(2):342-347.
- Brinton LA, Richesson DA, Gierach GL, et al. Prospective evaluation of risk factors for male breast cancer. J Natl Cancer Inst. 2008;100(20):1477-1481. doi:10.1093/jnci/djn329
- Rizzolo P, Silvestri V, Tommasi S, et al. Male breast cancer: genetics, epigenetics, and ethical aspects. Ann Oncol. 2013;24 Suppl 8:viii75-viii82. doi:10.1093/annonc/mdt316
- Friedman LS, Gayther SA, Kurosaki T, et al. Mutation analysis of BRCA1 and BRCA2 in a male breast cancer population. Am J Hum Genet. 1997;60(2):313-319.
- Breast Cancer Linkage Consortium. Cancer risks in BRCA2 mutation carriers. J Natl Cancer Inst. 1999;91(15):1310-1316. doi:10.1093/jnci/91.15.1310
- Thompson D, Easton DF; Breast Cancer Linkage Consortium. Cancer incidence in BRCA1 mutation carriers. J Natl Cancer Inst. 2002;94(18):1358-1365. doi:10.1093/jnci/94.18.1358
- Tai YC, Domchek S, Parmigiani G, Chen S. Breast cancer risk among male BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 2007;99(23):1811-1814. doi:10.1093/jnci/djm203
- Evans DGR, Susnerwala I, Dawson J, Woodward E, Maher ER, Lalloo F. Risk of breast cancer in male BRCA2 carriers. J Med Genet. 2010;47(10):710-711. doi:10.1136/jmg.2009.075176
- Meijers-Heijboer H, van den Ouweland A, Klijn J, et al; CHEK2-Breast Cancer Consortium. Low-penetrance susceptibility to breast cancer due to CHEK2(*)1100delC in noncarriers of BRCA1 or BRCA2 mutations. Nat Genet. 2002;31(1):55-59. doi:10.1038/ng879
- Fackenthal JD, Marsh DJ, Richardson AL, et al. Male breast cancer in Cowden syndrome patients with germline PTEN mutations. J Med Genet. 2001;38(3):159-164. doi:10.1136/jmg.38.3.159
- Silvestri V, Rizzolo P, Zanna I, et al. PALB2 mutations in male breast cancer: a population-based study in Central Italy. Breast Cancer Res Treat. 2010;122(1):299-301. doi:10.1007/s10549-010-0797-z
- Boyd J, Rhei E, Federici MG, et al. Male breast cancer in the hereditary nonpolyposis colorectal cancer syndrome. Breast Cancer Res Treat. 1999;53(1):87-91. doi:10.1023/a:1006030116357
- Wooster R, Mangion J, Eeles R, et al. A germline mutation in the androgen receptor gene in two brothers with breast cancer and Reifenstein syndrome. Nat Genet. 1992;2(2):132-134. doi:10.1038/ng1092-132
- Thomas DB, Rosenblatt K, Jimenez LM, et al. Ionizing radiation and breast cancer in men (United States). Cancer Causes Control. 1994;5(1):9-14. doi:10.1007/BF01830721
- Brinton LA, Cook MB, McCormack V, et al. Anthropometric and hormonal risk factors for male breast cancer: male breast cancer pooling project results. J Natl Cancer Inst. 2014;106(3):djt465. doi:10.1093/jnci/djt465. Published correction appears in J Natl Cancer Inst. 2014;106(5):dju117.
- Thomas DB, Jimenez LM, McTiernan A, et al. Breast cancer in men: risk factors with hormonal implications. Am J Epidemiol. 1992;135(7):734-748. doi:10.1093/oxfordjournals.aje.a116360
- Hultborn R, Hanson C, Köpf I, Verbiené I, Warnhammar E, Weimarck A. Prevalence of Klinefelter’s syndrome in male breast cancer patients. Anticancer Res. 1997;17(6D):4293-4297.
- Goss PE, Reid C, Pintilie M, Lim R, Miller N. Male breast carcinoma: a review of 229 patients who presented to the Princess Margaret Hospital during 40 years: 1955-1996. Cancer. 1999;85(3):629-639. doi:10.1002/(sici)1097-0142(19990201)85:3<629::aid-cncr13>3.0.co;2-v
- Expert Panel on Breast Imaging; Niell BL, Lourenco AP, Moy L, et al. ACR Appropriateness Criteria® evaluation of the symptomatic male breast. J Am Coll Radiol. 2018;15(11S):S313-S320. doi:10.1016/j.jacr.2018.09.017
- Greif JM, Pezzi CM, Klimberg VS, Bailey L, Zuraek M. Gender differences in breast cancer: analysis of 13,000 breast cancers in men from the National Cancer Data Base. Ann Surg Oncol. 2012;19(10):3199-3204. doi:10.1245/s10434-012-2479-z
- Yen PPW, Sinha N, Barnes PJ, Butt R, Iles S. Benign and malignant male breast diseases: radiologic and pathologic correlation. Can Assoc Radiol J. 2015;66(3):198-207. doi:10.1016/j.carj.2015.01.002
- Günhan-Bilgen I, Bozkaya H, Ustün EE, Memiş A. Male breast disease: clinical, mammographic, and ultrasonographic features. Eur J Radiol. 2002;43(3):246-255. doi:10.1016/s0720-048x(01)00483-1
- Stalsberg H, Thomas DB, Rosenblatt KA, et al. Histologic types and hormone receptors in breast cancer in men: a population-based study in 282 United States men. Cancer Causes Control. 1993;4(2):143-151. doi:10.1007/BF00053155
- Anderson WF, Devesa SS. In situ male breast carcinoma in the Surveillance, Epidemiology, and End Results database of the National Cancer Institute. Cancer. 2005;104(8):1733-1741. doi:10.1002/cncr.21353
- Vermeulen MA, Slaets L, Cardoso F, et al. Pathological characterisation of male breast cancer: results of the EORTC 10085/TBCRC/BIG/NABCG International Male Breast Cancer Program. Eur J Cancer. 2017;82:219-227. doi:10.1016/j.ejca.2017.01.034
- Cardoso F, Bartlett JMS, Slaets L, et al. Characterization of male breast cancer: results of the EORTC 10085/TBCRC/BIG/NABCG International Male Breast Cancer Program. Ann Oncol. 2018;29(2):405-417. doi:10.1093/annonc/mdx651
- Piscuoglio S, Ng CKY, Murray MP, et al. The genomic landscape of male breast cancers. Clin Cancer Res. 2016;22(16):4045-4056. doi:10.1158/1078-0432.CCR-15-2840
- Johansson I, Nilsson C, Berglund P, et al. Gene expression profiling of primary male breast cancers reveals two unique subgroups and identifies N-acetyltransferase-1 (NAT1) as a novel prognostic biomarker. Breast Cancer Res. 2012;14(1):R31. doi:10.1186/bcr3116
- Leone JP, Leone J, Zwenger AO, Iturbe J, Leone BA, Vallejo CT. Locoregional treatment and overall survival of men with T1a,b,cN0M0 breast cancer: a population-based study. Eur J Cancer. 2017;71:7-14. doi:10.1016/j.ejca.2016.10.038
- Cloyd JM, Hernandez-Boussard T, Wapnir IL. Outcomes of partial mastectomy in male breast cancer patients: analysis of SEER, 1983-2009. Ann Surg Oncol. 2013;20(5):1545-1550. doi:10.1245/s10434-013-2918-5
- Zaenger D, Rabatic BM, Dasher B, Mourad WF. Is breast conserving therapy a safe modality for early-stage male breast cancer?. Clin Breast Cancer. 2016;16(2):101-104. doi:10.1016/j.clbc.2015.11.005
- Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347(16):1227-1232. doi:10.1056/NEJMoa020989
- Gentilini O, Chagas E, Zurrida S, et al. Sentinel lymph node biopsy in male patients with early breast cancer. Oncologist. 2007;12(5):512-515. doi:10.1634/theoncologist.12-5-512
- Flynn LW, Park J, Patil SM, Cody HS III, Port ER. Sentinel lymph node biopsy is successful and accurate in male breast carcinoma. J Am Coll Surg. 2008;206(4):616-621. doi:10.1016/j.jamcollsurg.2007.11.005
- Whelan TJ, Pignol J-P, Levine MN, et al. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med. 2010;362(6):513-520. doi:10.1056/NEJMoa0906260
- Haviland JS, Owen JR, Dewar JA, et al; START Trialists’ Group. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013;14(11):1086-1094. doi:10.1016/S1470-2045(13)70386-3
- Abrams MJ, Koffer PP, Wazer DE, Hepel JT. Postmastectomy radiation therapy is associated with improved survival in node-positive male breast cancer: a population analysis. Int J Radiat Oncol Biol Phys. 2017;98(2):384-391. doi:10.1016/j.ijrobp.2017.02.007
- Madden NA, Macdonald OK, Call JA, Schomas DA, Lee CM, Patel S. Radiotherapy and male breast cancer: a population-based registry analysis. Am J Clin Oncol. 2016;39(5):458-462. doi:10.1097/COC.0000000000000078
- Eggemann H, Ignatov A, Stabenow R, et al. Male breast cancer: 20-year survival data for post-mastectomy radiotherapy. Breast Care (Basel). 2013;8(4):270-275. doi:10.1159/000354122
- Jardel, PVignot S, Cutuli B, et al.Shoudl adjuvant therapy be systemically proposed for male breast cancer ? a systematic review. Anticancer Research 2018;38:23-31. doi:10.21873/anticanres.12187
- Yadav S, Karam D, Bin Riaz I, et al. Male breast cancer in the United States: treatment patterns and prognostic factors in the 21st century. Cancer. 2020;126(1):26-36. doi:10.1002/cncr.32472
- Walshe JM, Berman AW, Vatas U, et al. A prospective study of adjuvant CMF in males with node positive breast cancer: 20-year follow-up. Breast Cancer Res Treat. 2007;103(2):177-183. doi:10.1007/s10549-006-9363-0
- Giordano SH, Perkins GH, Broglio K, et al. Adjuvant systemic therapy for male breast carcinoma. Cancer. 2005;104(11):2359-2364. doi:10.1002/cncr.21526
- Izquierdo MA, Alonso C, De Andres L, Ojeda B. Male breast cancer. report of a series of 50 cases. Acta Oncol. 1994;33(7):767-771. doi:10.3109/02841869409083946
- Patel HZ II, Buzdar AU, Hortobagyi GN. Role of adjuvant chemotherapy in male breast cancer. Cancer. 1989;64(8):1583-1585. doi:10.1002/1097-0142(19891015)64:8<1583::aid-cncr2820640804>3.0.co;2-q
- Yildirim E, Berberoğlu U. Male breast cancer: a 22-year experience. Eur J Surg Oncol. 1998;24(6):548-552. doi:10.1016/s0748-7983(98)93608-3
- Ribeiro GG, Swindell R, Harris M, Banerjee SS, Cramer A. A review of the management of the male breast carcinoma based on an analysis of 420 treated cases. Breast. 1996;5(3):141-146. doi:10.1016/S0960-9776(96)90058-2
- Eggemann H, Ignatov A, Smith BJ, et al. Adjuvant therapy with tamoxifen compared to aromatase inhibitors for 257 male breast cancer patients. Breast Cancer Res Treat. 2013;137(2):465-470. doi:10.1007/s10549-012-2355-3
- Harlan LC, Zujewski JA, Goodman MT, Stevens JL. Breast cancer in men in the United States: a population-based study of diagnosis, treatment, and survival. Cancer. 2010;116(15):3558-3568. doi:10.1002/cncr.25153
- Mauras N, O’Brien KO, Klein KO, Hayes V. Estrogen suppression in males: metabolic effects. J Clin Endocrinol Metab. 2000;85(7):2370-2377. doi:10.1210/jcem.85.7.6676
- Hayes FJ, Seminara SB, Decruz S, Boepple PA, Crowley WF Jr. Aromatase inhibition in the human male reveals a hypothalamic site of estrogen feedback. J Clin Endocrinol Metab. 2000;85(9):3027-3035. doi:10.1210/jcem.85.9.6795
- Hassett MJ, Somerfield MR, Giordano SH. Management of male breast cancer: ASCO guideline summary. JCO Oncol Pract. 2020;16(8):e839-e843. doi:10.1200/JOP.19.00792
- Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351(27):2817-2826. doi:10.1056/NEJMoa041588
- Crozier J, Barone J, Banda K, et al; FLEX Investigators Group. Differential gene expression and clinical utility of MammaPrint and BluePrint in male breast cancer patients. Cancer Res. 2021;81(4):abstr PS14-11. doi:10.1158/1538-7445.SABCS20-PS14-11
- Massarweh SA, Sledge GW, Miller DP, McCullough D, Petkov VI, Shak S. Molecular characterization and mortality from breast cancer in men. J Clin Oncol. 2018;36(14):1396-1404. doi:10.1200/JCO.2017.76.8861
- Grenader T, Yerushalmi R, Tokar M, et al. The 21-gene recurrence score assay (Oncotype DX™) in estrogen receptor–positive male breast cancer: experience in an Israeli cohort. Oncology. 2014;87(1):1-6. doi:10.1159/000360793
- Dubrovsky E, Raymond S, Chun J, Schnabel F. Gene expression profiling in male breast cancer. Cancer Res. 2018;78(4):abstr P5-23-03. doi:10.1158/1538-7445.SABCS17-P5-23-03
- De Leeneer K, Coene I, Crombez B, et al. Prevalence of BRCA1/2 mutations in sporadic breast/ovarian cancer patients and identification of a novel de novo BRCA1 mutation in a patient diagnosed with late onset breast and ovarian cancer: implications for genetic testing. Breast Cancer Res Treat. 2012;132(1):87-95. doi:10.1007/s10549-011-1544-9
- Petrucelli N, Daly MB, Pal T. BRCA1- and BRCA2-associated hereditary breast and ovarian cancer. In: Adam MP, Ardinger HH, Pagon RA, et al, eds. GeneReviews®. University of Washington, Seattle; September 4, 1998. Updated December 15, 2016. Accessed July 28,2021. https://pubmed.ncbi.nlm.nih.gov/20301425/
- Offit K, Levran O, Mullaney B, et al. Shared genetic susceptibility to breast cancer, brain tumors, and Fanconi anemia. J Natl Cancer Inst. 2003;95(20):1548-1551. doi:10.1093/jnci/djg072
- Sawyer SL, Tian L, Kähkönen M, et al; University of Washington Centre for Mendelian Genomics; FORGE Canada Consortium. Biallelic mutations in BRCA1 cause a new Fanconi anemia subtype. Cancer Discov. 2015;5(2):135-142. doi:10.1158/2159-8290.CD-14-1156
- Whittemore AS, Gong G, John EM, et al. Prevalence of BRCA1 mutation carriers among U.S. non-Hispanic Whites. Cancer Epidemiol Biomarkers Prev. 2004;13(12):2078-2083.
- Graeser MK, Engel C, Rhiem K, et al. Contralateral breast cancer risk in BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2009;27(35):5887-5892. doi:10.1200/JCO.2008.19.9430
- Liede A, Karlan BY, Narod SA. Cancer risks for male carriers of germline mutations in BRCA1 or BRCA2: a review of the literature. J Clin Oncol. 2004;22(4):735-742. doi:10.1200/JCO.2004.05.055
- Kuchenbaecker KB, Hopper JL, Barnes DR, et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA. 2017;317(23):2402-2416. doi:10.1001/jama.2017.7112
- NCCN. Clinical Practice Guidelines in Oncology. Genetic/familial high-risk assessment: breast, ovarian, and pancreatic cancer, version 1.2021. Accessed July 28.2021. https://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf
- Jemal A, Lin CC, DeSantis C, Sineshaw H, Freedman RA. Temporal trends in and factors associated with contralateral prophylactic mastectomy among US men with breast cancer. JAMA Surg. 2015;150(12):1192-1194. doi:10.1001/jamasurg.2015.2657

Oncology Peer Review On-The-Go: COVID-19, Cancer, and the Potential of mRNA Vaccines
March 30th 2021Mehmet Sitki Copur, MD, discussed his article in the Journal ONCOLOGY® focusing on COVID-19, messenger RNA vaccines, and the excitement surrounding its integration into the future of cancer treatment.