Articles by Burton L. Eisenberg, MD
ABSTRACT
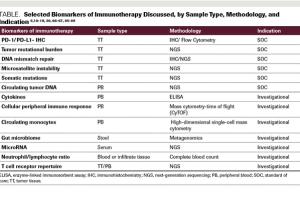
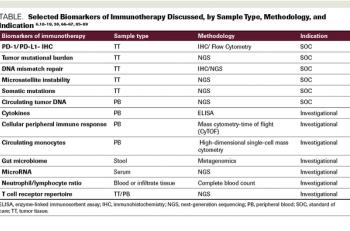
Advances in immuno-oncology over the last several years have led to FDA approvals of novel agents. As our understanding of immune response and its checkpoints has evolved, further advances have been made in treatment for several cancer types. To predict a response to immunotherapy, the initial biomarkers used were expression of the PD-1 receptor and PD-L1, as assessed by immunohistochemistry. More recently, predictive biomarkers have included microsatellite instability, DNA mismatch repair, and tumor mutational burden. Although these markers may be clinically relevant in predicting an immunotherapy response, cancer immunotherapy fails some patients. Improved understanding of the human immune system is necessary, as is a careful evaluation of the methods used to predict and assess response to Immuno-oncology treatments. With the application of therapeutic immune-modulating agents, more comprehensive assays, and associated bioinformatics tools to accurately assess the tumor microenvironment, we may better predict responses to immuno-oncology agents and the ever-increasing complexity of their clinical use.
Although their overall incidence is uncommon, gastrointestinal stromaltumors (GIST) are the most frequently encountered mesenchymaltumors of the GI tract. Their pathology has been recently defined bythe presence of KIT (transmembrane receptor tyrosine kinase). Themajority of GISTs have c-kit gain-of-function mutations mainly in exon11 (highly conserved juxtamembrane region) that eventuates in constitutiveactivation of KIT, promoting proliferation and antiapoptotic signaling.Imatinib mesylate (Gleevec) is a specific inhibitor of KIT kinaseactivation, and in phase II clinical trials has proven to be remarkablyefficacious in heavily pretreated GIST patients with advanced disease.The molecular and genomic determinants of response/resistancepatterns are the subject of ongoing studies, and adjuvant studies arealso under way. The initial evaluations of imatinib provide proof ofconcept for the hypothesis-driven design of selective molecularly targetedtherapies for solid tumor malignancies.

Over the past 7 years, 58 saline-filled tissues expanders (TEs) have been temporarily placed in 57 patients. The

Soft-tissue sarcomas arising from the retroperitoneum are rare tumors, and their successful treatment is problematic. This group of tumors tends to be large at presentation, and they exist in a body cavity with few fascial planes to contain them. They frequently abut vital organs or major blood vessels, which further complicates their complete removal.

Total pelvic exenteration is a radical abdominoperineal operation designed to treat locally extensive pelvic malignancy. In the past, the morbidity and mortality has been such that this procedure was considered justified