Colorectal cancer is one of the most commonly diagnosed cancers in the US, and it is the second leading cause of cancer-related deaths. The risk of developing colorectal cancer increases with age.

Your AI-Trained Oncology Knowledge Connection!


Colorectal cancer is one of the most commonly diagnosed cancers in the US, and it is the second leading cause of cancer-related deaths. The risk of developing colorectal cancer increases with age.

An estimated 250 million people globally, and 25 million in the US, suffer from rare diseases. Clearly, because of their low incidence, rare cancers are difficult to study and pose challenges for detection, diagnosis, prognosis, and treatment.

The Swiss pharmaceutical company Roche and Roche Group member Genentech have announced that addition of bevacizumab (Avastin) to chemotherapy improved progression-free survival over chemotherapy alone in the phase III OCEANS ovarian cancer study, meeting the study’s primary endpoint.
Questions over safety in Delcath Systems' chemotherapy delivery system for melphalan in the treatment of liver tumors caused the FDA to issue a refusal-to-file letter.

The American Society for Radiation Oncology (ASTRO) has released guidelines for the use of radiation therapy in treating bone metastases; the guidelines are published in the International Journal of Radiation Oncology, Biology, Physics.
While more than 12 million people in the US are cancer survivors, an online survey conducted by the Oncology Nursing Society (ONS) has found that only about one-quarter of oncology nurses surveyed had a formal survivorship program in place at their institutions.

A team of researchers at Stony Brook University School of Medicine in New York published a paper in JAMA last week showing that patients who receive Avastin (bevacizumab), in combination with chemotherapy are at increased risk of side effects that may lead to death

A new law, introduced by Senators Amy Klobuchar (D-Minnesota) and Bob Casey (D-Pennsylvania), will require prescription drug manufacturers to give the US Food and Drug Administration (FDA) early warning of anything that will likely result in a drug shortage.

The FDA has approved Rituxan for first-line maintenance treatment of patients with advanced follicular lymphoma who responded to initial treatment with Rituxan plus chemotherapy.

The FDA has just officially released rough guidelines for use by industry for the development of two or more novel investigational drugs, for use in combination.
The GlaxoSmithKline (GSK) drug Avodart (dutasteride), already approved for treatment in men with enlarged prostate glands, has been rejected by the FDA for the additional indication of reducing the risk of prostate cancer.

A recent analysis in the Cancer Journal outlines the various effects of last year's landmark health care reform bill on cancer research. Some positive changes will be in the pipeline as will some potentially negative unanticipated consequences.

According to a new study by researchers at the Fred Hutchinson Cancer Research Center, postmenopausal women who experience hot flushes and other menopause symptoms may have a 50% lower risk of developing the most common forms of breast cancer.

Recent data from the National Center for Health Statistics (NCHS) shows that an increasing number of primary care physicians have already adopted a basic EHR, but most physicians would need to further upgrade their EHR systems or their use of the systems in order to qualify for “meaningful use” incentive payments.

A new study published in the Archives of Internal Medicine shows that smokers are more likely to develop breast cancer than nonsmokers.

ASCO’s QOPI analysis recently found that less than half of all cancer patients are enrolled in hospice care before their death and of those who are enrolled, one-third are not enrolled until the last week of their lives. In response to this and other findings, ASCO published a new policy and guidelines this week, intended to encourage physicians to initiate open dialogue about palliative care and treatment with patients as soon as they are identified as having incurable cancer.

Researchers at the NCI reported today in Nature that interferon-gamma, a protein that had been thought to contribute to an innate defense system against cancer, may in some circumstances promote melanoma and incite the development of tumors.
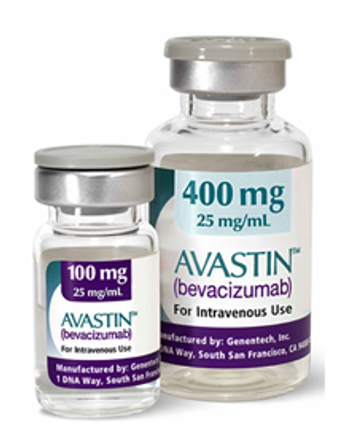
Genentech, owner of Roche, submitted its response yesterday to the FDA’s proposal to withdraw approval for Avastin (bevacizumab) for the treatment of HER2-negative metastatic breast cancer.

According to a recent analysis by researchers at the National Cancer Institute, in 2020, medical expenditures for cancer should reach at least $158 billion (in 2010 dollars). This represents an increase of 27 percent over 2010.