The CheckMate 511 phase IIIb/IV trial investigated raising the nivolumab dose to 3 mg/kg and lowering the ipilimumab dose to 1 mg/kg.

Your AI-Trained Oncology Knowledge Connection!


The CheckMate 511 phase IIIb/IV trial investigated raising the nivolumab dose to 3 mg/kg and lowering the ipilimumab dose to 1 mg/kg.

The US Food and Drug Administration explored the possibility of such bias in a recent viewpoint published in JAMA Oncology.
The latest trial results are based on a larger patient population and were reported at the 2019 ASCO Genitourinary Cancers Symposium.
The results of the double-blind, phase III trial were presented at the 2019 ASCO Genitourinary Cancers Symposium.
An exploratory analysis showed that patients who received nivolumab plus ipilimumab had better patient-reported outcomes. Do the results hold up?

Researchers identified two germline pathogenic variants in CHEK2 that may account for a minority of men diagnosed with testicular germ cell tumors.
Results of the 2-year follow-up analysis of the pivotal KEYNOTE-024 trial were recently published in the Journal of Clinical Oncology.
The study, published in JAMA Oncology, examined whether treatment of 22 solid tumor types was associated with two therapy-related conditions.
An analysis of a large, phase III trial investigated a potential surrogate endpoint for survival outcomes in prostate cancer patients receiving ADT.

The results of the study add to the growing body of literature regarding potential biomarkers for response to immune checkpoint inhibitors.
The results, described by an expert as “very interesting” and a “a bit surprising," focused on the impact of autoimmune antibodies on anti–PD-1 therapy.
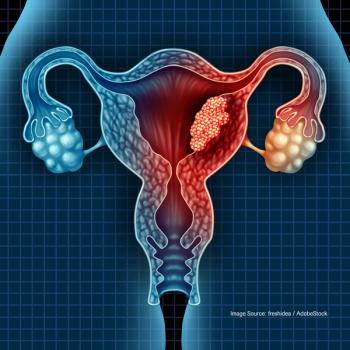
Experts weigh in on valuable findings that can be gleaned despite the fact that the phase III trial did not generate clinically meaningful results.
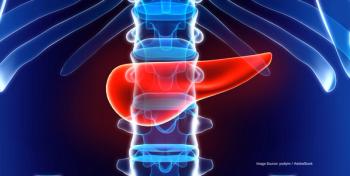
Investigators evaluated whether patients with pancreatic ductal adenocarcinoma benefit from adjuvant therapy after surgery if the tumor is smaller than 1 cm.
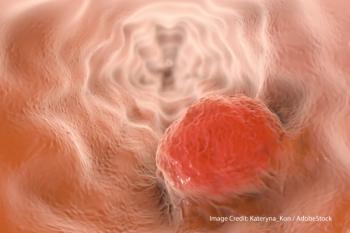
A large pooled analysis of individuals with advanced esophageal cancer showed men and women have different side effects to chemotherapy.

A recent study in JAMA Oncology examined the average daily cost of biosimilar filgrastim-sndz compared with the brand name biologic.