
Data from the EPIK-B5 study support the use of alpelisib/fulvestrant in previously treated, HR-positive, HER2-negative, PIK3CA-mutated breast cancer.

Your AI-Trained Oncology Knowledge Connection!


Data from the EPIK-B5 study support the use of alpelisib/fulvestrant in previously treated, HR-positive, HER2-negative, PIK3CA-mutated breast cancer.
Data from a phase 1/2 trial support sonrotoclax as a promising treatment option in previously treated relapsed/refractory mantle cell lymphoma.

The CR/CR with incomplete bone marrow recovery rate was 12.8% in patients with relapsed/refractory CLL/SLL following zanubrutinib treatment.

An ORR of 91.4% was observed with zanuburtinib plus R-CHOP in patients with DLBCL.
The confirmed ORR in the investigational arm was 52.3% vs 46.6% in the chemotherapy arm, with respective complete response rates of 10.9% and 8.5%.

In terms of OS among patients with non-tBRCA–mutated, HRD-positive disease, the median value was not reached in either durvalumab arm.

Nine-year final results from the CheckMate 238 trial demonstrated that adjuvant nivolumab significantly improved time to second disease progression.

Adjuvant ribociclib plus aromatase inhibitors significantly improved invasive disease-free survival in early breast cancer, as shown in the NATALEE trial.

The median CNS PFS with Dato-DXd was 5.0 months vs 3.0 months with docetaxel in patients with NSCLC who have brain metastases.
A new biomarker, KIM-1, has the potential to show outcomes and response for patients with renal cell carcinoma.
Data from the phase 3b ProvIDHe trial show "reassuringly good" PFS and OS outcomes with ivosidenib in a real-world cohort.
There appeared to be no extra benefit with the addition of nivolumab to tivozanib among patients included in the phase 3 TiNivo-2 trial.
Fruquintinib plus chemotherapy and a PD-1 inhibitor elicited an ORR of 80.0% with all partial responses in patients with treatment-naïve gastric/GEJ cancer.

An efficacy advantage with osimertinib-containing regimens was consistent across predefined patient subgroups in those with EGFR-mutant NSCLC.

Data from SACHI support savolitinib/osimertinib as a chemotherapy-free option in previously treated EGFR-mutated NSCLC harboring a MET amplification.

Patients who received ipatasertib/fulvestrant in the intention-to-treat population achieved a median PFS of 5.32 months compared with 1.94 months in the placebo arm.

The ARANOTE phase 3 trial showed darolutamide plus ADT improved outcomes in mHSPC regardless of disease volume.

NFKB1 rs230511 genetic variant improves treatment outcomes for patients with polycythemia vera and essential thrombocythemia.

Patients aged 60 to 69 years old had comparable efficacy when treated with brexu-cel for relapsed/refractory B-cell ALL.

Treatment with THIO/cemiplimab was generally well-tolerated among patients with advanced NSCLC in the phase 2 THIO-101 study.

Phase 3 data support benmelstobart/anlotinib as a new potential standard in the frontline treatment of patients with advanced renal cell carcinoma.

A median EFS of 40.1 months was noted in patients resectable NSCLC given perioperative nivolumab vs placebo.

The primary end point of progression-free survival was met with retifanlimab to carboplatin and paclitaxel in patients with metastatic SCAC.

Data from the phase 3b JUMP trial support the use of ruxolitinib in myelofibrosis regardless of baseline or treatment-related anemia.

Additionally, the 48-month overall survival rate was higher with nivolumab/cabozantinib vs sunitinib in the phase 3 CheckMate 9ER trial.
“Our analysis showed that the time to progression was later with the use of lenvatinib and pembrolizumab compared with sunitinib,” said Viktor Grünwald, MD, PhD.
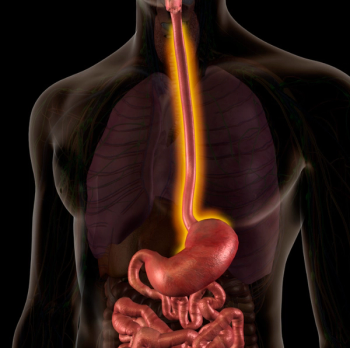
The combination of perioperative chemotherapy plus FLOT improved overall survival vs neoadjuvant chemoradiation in patients with resectable esophageal cancer.

The benefit-risk profile of asciminib may change the chronic myeloid leukemia treatment paradigm, according to Jorge E. Cortes, MD.

All patients with a response to TAR-200 did not have progression to muscle-invasive bladder cancer or metastatic disease in the SunRISe-1 trial.
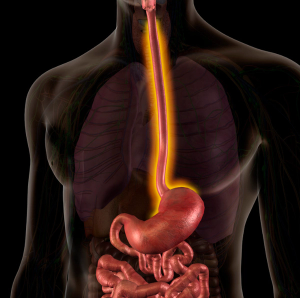
Published: June 2nd 2024 | Updated: June 3rd 2024
Published: December 12th 2023 | Updated:
Published: September 13th 2022 | Updated:
Published: September 10th 2022 | Updated:
Published: February 13th 2025 | Updated:
Published: December 11th 2023 | Updated: