
Patients with relapsed/refractory chronic lymphocytic leukemia or small lymphocytic lymphoma can now receive lisocabtagene maraleucel after the FDA approved the drug, which was based on the phase 1/2 TRANSCEND CLL trial.

Your AI-Trained Oncology Knowledge Connection!


Patients with relapsed/refractory chronic lymphocytic leukemia or small lymphocytic lymphoma can now receive lisocabtagene maraleucel after the FDA approved the drug, which was based on the phase 1/2 TRANSCEND CLL trial.
Imaging after 1 week of treatment with pembrolizumab may help identify treatment responses in patients with metastatic melanoma.

Starting April 1, 2024, Diane Simeone will be the new director of UCSD Moores Cancer Center.

The FDA will review the supplemental biologics license application for alectinib based on data from the phase 3 ALINA study.

Treatment with nivolumab did not reach the primary endpoint of disease-free survival when compared with placebo in patients with localized renal cell carcinoma at high risk of relapse after nephrectomy in the phase 3 CheckMate 914 trial.
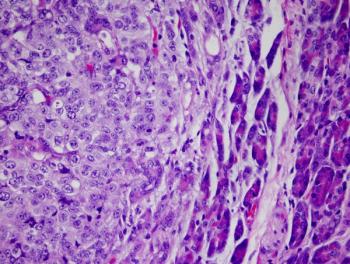
Supporting data for the EMA comes from the phase 3 EV-302 trial, which assessed the efficacy of enfortumab vedotin in combination with pembrolizumab compared with chemotherapy.
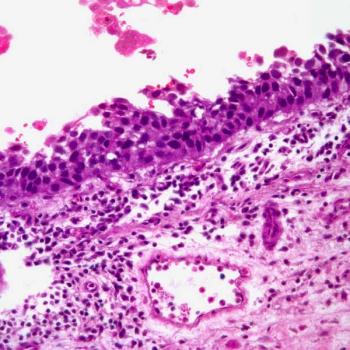
Risk-reducing total gastrectomy is associated with short-term and long-term physical and emotional AEs in patients with pathogenic or likely pathogenic germline CDH1 P/LP variants.

Treating patients with NSCLC with stereotactic ablative radiotherapy and chemotherapy reduces toxicity, according to a study conducted by UCLA.

In the overall population and in patients with a PD-L1 CPS of 5 or greater who received nivolumab plus chemotherapy, overall survival and progression-free survival improved when compared with chemotherapy alone.
Patients with advanced ovarian cancer who received primary or interval cytoreduction surgery experienced higher rates of overall and progression-free survival.

An analysis based on the phase 3 TRANSFORM and TRANSCEND trials shows an increase in cost-effectiveness, life-years, and quality-adjusted life-years compared with standard of care in patients with relapsed/refractory diffuse large B-cell lymphoma.






During the 2023 European Society of Medical Oncology, experts in the field of lung cancer convened to discuss the use of biomarker testing and updated trial results that were presented.

Clinicians discuss treatment options for advanced-stage multiple myeloma and consider the effects of renal disfunction.

After originally releasing the Medicare Prescription Drug Inflation Rebate Program in February 2023, CMS has issued changes which address ongoing drug shortages.
While the overall survival between patients receiving cabazitaxel and those receiving Lu-PSMA-617 had negligible difference, the results of the phase 2 TheraP trial supports the use of Lu-PSMA-617 as an alternative treatment for prostate cancer.
The FDA did not raise any concerns about the clinical data package, safety, or labeling for the approvability of cosibelimab for squamous cell carcinoma.

The safety profile of the phase 3 RELATIVITY-123 trial is not the cause of the discontinuation, as safety is consistent with prior trials assessing the combination.
The current drug supply shortage is due to manufacturing issues and economic factors, both of which can be addressed, according to Jason Westin, MD, MS, FACP.

Acalabrutinib on its own or in combination with obinutuzumab increases PFS and ORR in patients with chronic lymphocytic leukemia regardless of mutation status, according to follow-up data from the ELEVATE-TN trial.
Brentuximab vedotin plus nivolumab, doxorubicin, and dacarbazine appears to be well tolerated in patients with advanced stage classical Hodgkin lymphoma, according to data from the phase 2 SGN35-027 trial.

Data from the ZUMA-7 trial shows that age alone should not be a barrier of consideration of administering CAR T-cell therapy to patients with large B-cell lymphoma, says Marie José Kersten, MD.

Patients who are under the age of 17 with invasive mucormycosis or invasive aspergillosis, which is an AE of leukemia, can now receive isavuconazonium sulfate following FDA approval

The phase 3 NRG GY018 trial added immunotherapy to chemotherapy in patients with recurrent endometrial cancer, regardless of mismatch repair deficient or proficient disease status, according to Ramez N. Eskander, MD.

The DEBBRAH trial met its primary endpoint of overall survival in a cohort of patients with HER2-positive or HER2-low advanced breast cancer and leptomeningeal carcinomatosis with positive cerebrospinal fluid cytology.

Compared with surgery and radiation, focal therapy improves quality of life as well as decreases financial toxicity for patients with prostate cancer.
Published: December 1st 2023 | Updated:

Published: December 24th 2023 | Updated:
Published: January 3rd 2024 | Updated:

Published: December 10th 2023 | Updated:

Published: December 24th 2023 | Updated:
Published: December 17th 2023 | Updated: