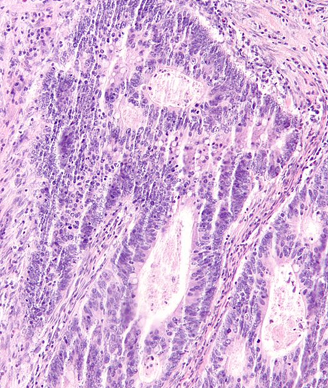
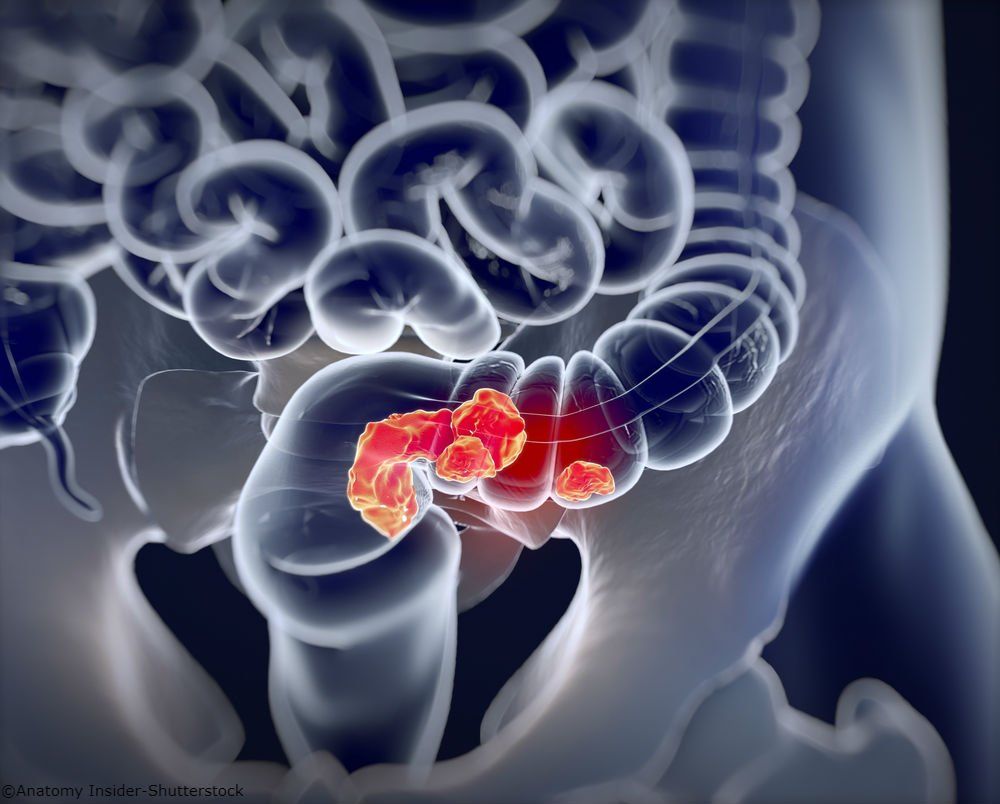
Colorectal Cancer
Latest News
Latest Videos

CME Content
More News

Real-world data suggest a role for noncytotoxic chemotherapy treatments such as EGFR inhibitors beyond the frontline setting for patients with metastatic colorectal cancer.

Results from the phase 3 CheckMate-8HW trial highlight that the safety of frontline nivolumab plus ipilimumab in microsatellite instability–high or mismatch repair deficient is comparable with prior reports.
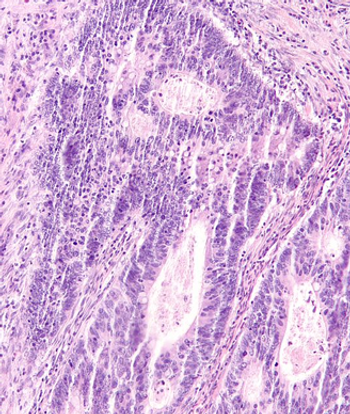
Real-world data may elucidate the characteristics and factors that influence long-term remission with regorafenib among patients with metastatic colorectal cancer.

Findings from the phase 3 FRESCO-2 trial support fruquintinib’s potential to provide an improved survival benefit and quality of life for those with previously treated metastatic colorectal cancer.
The DeFianCe trial is using DKN-01 plus bevacizumab and chemotherapy to determine if a clinical benefit would occur in patients with microsatellite stable colorectal adenocarcinoma.
Data demonstrate efficacy of robotic surgery for colectomies, which is swiftly becoming the 'preferred approach' for surgeons.

Kristen K. Ciombor, MD, MSCI, gives an overview of the current treatment landscape for patients with metastatic colorectal cancer.

The safety profile of the phase 3 RELATIVITY-123 trial is not the cause of the discontinuation, as safety is consistent with prior trials assessing the combination.
Colorectal cancer specialists discuss the effect of the SUNLIGHT trial data on the overall treatment and research landscapes.

New studies and treatment options prove to be effective in treating metastatic CRC, notes Joleen Hubbard, MD.

The safety profile of nivolumab plus ipilimumab in the phase 3 CheckMate 8HW trial is comparable with prior reports of each individual agent.

Experts on colorectal cancer provide their key takeaways from the results from the SUNLIGHT study.

A comprehensive review of outcome data from the SUNLIGHT clinical trial in patients with refractory metastatic colorectal cancer.

Marwan G. Fakih, MD, reviews the study design for the SUNLIGHT clinical trial investigating trifluridine-tipiracil plus bevacizumab in patients with refractory metastatic CRC.

A brief overview of treatment options available for patients with colorectal cancer prior to the SUNLIGHT study.

Personalized therapy tailored for mutation status is something to expect in the coming years for colorectal cancer treatment, notes Tanios S. Bekaii-Saab, MD.

Comprehensive insights on the stratification, diagnosis, and treatment of patients with metastatic colorectal cancer.

Marwan G. Fakih, MD, and Atif Hussein, MD, MMM, FACP, introduce themselves and give an overview of metastatic colorectal cancer (mCRC).

New treatment updates could help find more patient subtypes to target and treat in the world of CRC, according to Kristen K. Ciombor, MD, MSCI.

A study conducted by the American Cancer Society found patients who were Black received worse care for colorectal cancer treatment, with health insurance is the leading cause of racial disparities.

Drs Cohen, Wu, and Ahn discuss HER2 in colorectal cancer, explaining that increased expression indicates possible benefit from HER2 inhibitors; Ahn summarized studies showing modest activity of dual HER2 inhibition compared to single agent trastuzumab.

Drs Wu, Ciombor, and Cohen discussed upfront molecular testing in patients with colorectal cancer to guide treatment, with Dr Ciombor reviewing HER2 testing methods and Dr Cohen noting liquid biopsy limitations.

Arvind N. Dasari, MD, lead investigator of the FRESCO-2 trial, spoke about the recent approval of fruquintinib for patients with previously treated metastatic colorectal cancer.

Results from the phase 3 FRESCO-2 trial support the approval of fruquintinib for patients with previously treated metastatic colorectal cancer.
CHM 2101 will be assessed as part of a phase 1A/B clinical study in a population of patients diagnosed with advanced colorectal cancer, gastric cancer, and neuroendocrine cancer.