Results from an ongoing Phase II trial of patients with non-small cell lung cancer (NSCLC) that harbor a specific mutation in the anaplastic lymphoma kinase (ALK) gene reported at ASCO.

Your AI-Trained Oncology Knowledge Connection!


Results from an ongoing Phase II trial of patients with non-small cell lung cancer (NSCLC) that harbor a specific mutation in the anaplastic lymphoma kinase (ALK) gene reported at ASCO.

Lung cancer remains the leading cause of cancer-related death in the United States. Ongoing research into the molecular basis of lung cancer has yielded insight into various critical pathways that are deregulated in lung tumorigenesis, and in particular key driver mutations integral to cancer cell survival and proliferation.

In 2004, Dr. Thomas Lynch[1] and others[2] first reported the presence of somatic mutations in the epidermal growth factor receptor (EGFR) gene in patients who exhibited great sensitivity to EGFR tyrosine kinase inhibitors (TKIs).

The authors of "ALK-Targeted Therapy for Lung Cancer: Ready for Prime Time," in this issue of ONCOLOGY, address the newest developments in the field of targeted therapies for advanced non–small-cell lung cancer (NSCLC).

Researchers from Memorial Sloan Kettering Cancer Center and the Weill Medical School of Cornell University have shown an association of epidermal growth factor receptor (EGFR) mutations among tumor samples from men and those who smoke cigarettes.
Researchers at UCLA have engineered “vaults,” barrel-shaped nanoscale capsules found in the cytoplasm of mammalian cells, to slowly release chemokine CCL21 into tumors. CCL21 is a protein that, in pre-clinical studies in mice with lung cancer, stimulated the immune system to recognize and attack the cancer cells.

We review the evidence implicating a strong association between chronic inflammation and cancer, with an emphasis on colorectal and lung cancer.
A team of researchers has used mass spectrometry to identify a novel six-biomarker serum test that effectively identified lung cancer in never smokers, and which may have other important diagnostic applications in lung cancer.
Erlotinib (Tarceva) has a low response rate in non–small cell lung cancer (NSCLC) but does improve survival in a subpopulation of patients harboring a wild-type (wt) epidermal growth factor receptor (EGFR) gene.
“Hallmarks of Cancer”, published in the journal Cell in 2000 provided a conceptual framework for the evolution of cancer as well as an all-encompassing review of the cancer field to date. The article is updated in the March 4th, 2011 issue of Cell.

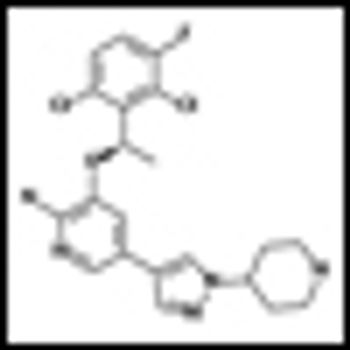
Pfizer’s crizotinib, an oral, selective, small-molecule inhibitor for non-small cell lung cancer (NSCLC) has gone remarkably fast from lab bench to late-stage trials and now to the FDA for review.

“How long have I had this cancer, Doctor?” This is a question that patients frequently ask their oncologist.

In their article, Patrone et al utilize a modified version of Collins’ law to estimate the age of breast, lung, and colorectal cancers. Collins’ law, which states that the period of risk for recurrence of a tumor is equal to the age of the patient at diagnosis plus 9 months, has been applied primarily to pediatric tumors, in particular embryonal tumors.[1,2] The results from the application of Collins’ law to these tumors have been reasonable, although exceptions have been reported and the law is not applicable to all cancers.[3,4] Its utilization in adults in the manner used in this paper is therefore unique.
Exciting advances in understanding the biology of lung cancer have occurred over the last few years.

The authors of “How Long Have I Had My Cancer, Doctor?” have addressed a question often contemplated by patients when they receive a diagnosis of cancer.

The choices that patients and clinicians make when dealing with cancer are dictated by time, whether they are arranging for screening mammography and colonoscopy, compiling treatment plans, or determining follow-up intervals and the age of freedom from follow-up.

Ganti and colleagues have provided a comprehensive overview on recent presentations involving lung cancer.

Ganti et al have described the most recent negative lung cancer chemoprevention trial, which compared selenium to placebo.

The number of patients in the U.S. treated with radiation has increased at an average annual rate of about 7% between 2007 and 2009, according to the “2010 Radiation Therapy Market Summary Report” by IMV. Breast, prostate, and lung cancers continue to be the cancer types treated most frequently with radiation.

Patients with advanced non-small-cell lung cancer achieved a significant increase in survival time when tumor treating fields (TTF) therapy was added to their chemotherapy. In a single-arm, phase II study, physicians delivered TTF therapy, using the NovoTTF-100L from Novocure, to 42 patients with stage IIIb-IV metastatic NSCLC who had failed prior treatments. Patients in the study received TTF therapy for 12 hours a day in combination with pemetrexed (Alimta) every three weeks until disease progression.

Finding an effective treatment for all the complex iterations of cancer is akin to chasing an outlaw through a treacherous mountain range, in the estimation of Louis M. Weiner, MD, director of the Georgetown Lombardi Comprehensive Cancer Center in Washington, DC.

Research from Japan documenting remarkable survival rates among patients with inoperable lung cancer may only hint at the potential of proton-beam radiation therapy. The study out of the Proton Medical Research Center in Tennoudai, Japan, documented high survival rates for 55 patients suffering from stage I inoperable non-small-cell lung cancer.

Novartis and its partner Antisoma announced that an interim analysis of data from a late-stage trial showed that vadimezam (ASA404) was unlikely to provide any benefit as a second-line treatment for patients with non-small-cell lung cancer. As a result, Novartis indicated that it would halt development of the compound, which was designed to inhibit angiogenesis.

The key to effective treatment of squamous lung cell lung cancer in smokers may be at hand.
Patients with incurable NSCLC are less likely to progress to second-linetherapy with the right maintenance regimen. But maintenance therapyalso means committing patients to continuous treatment without anybreaks or chances to recover from adverse events.