Radiation Oncology
Latest News

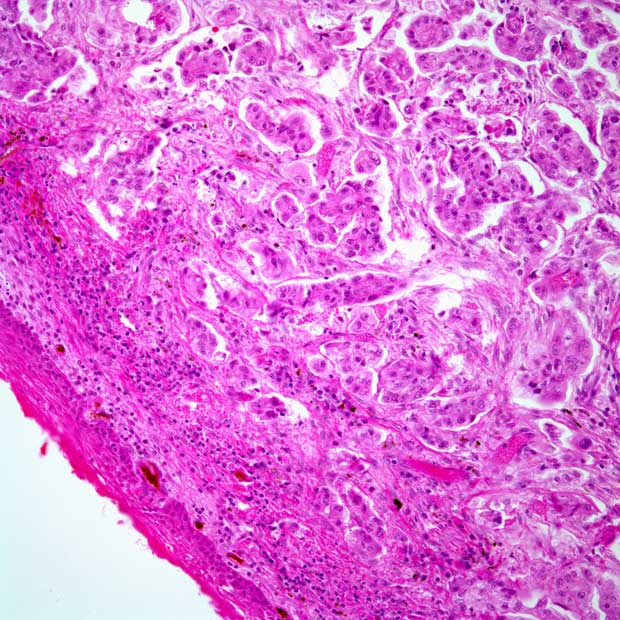
Lapatinib Does Not Improve CRT Outcomes in Non-HPV Head and Neck Carcinoma
Yoga May Reduce Physical Decline in HNC Population Receiving Radiotherapy
Latest Videos

CME Content
More News

Those with breast cancer who have undergone implant-based reconstruction following mastectomy have similar outcomes with hypofractionated vs conventionally fractionated radiotherapy.

Diagnostic CT-enabled radiation therapy also reduces patient-reported time burden in the palliative setting.

Data from the FABREC trial support the use of hypofractionated postmastectomy radiotherapy in patients with breast cancer following implant-based reconstruction.

Real-world data suggest that the presence of pelvic lymphadenopathy should not be viewed as a contraindication to trimodality therapy in nonmetastatic clinically node-positive bladder cancer.

Jonathan Leeman, MD, indicates that the longer-follow is necessary to confirm late toxicities and disease control associated with adaptive radiotherapy in patients with prostate cancer.

Increasing radiation doses to the whole heart appear to correlate with higher risks of valvular disease, coronary artery disease, and heart failure in childhood cancer survivors.

Cutting out radiotherapy appears to be safe while improving quality of life in patients with POLE-mutated endometrial cancer.

The FDA requires data from an additional clinical trial to support the potential approval of avasopasem for managing radiation-induced severe oral mucositis in patients with head and neck cancer.
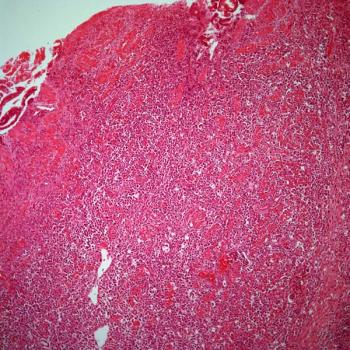
The addition of stereotactic radiotherapy to immune checkpoint inhibition appears safe but yields no significant survival benefit among patients with advanced solid tumors.

Fifteen-year follow-up data suggest the importance of considering the trade-offs between risks and benefits of active monitoring, prostatectomy, and radiotherapy for localized prostate cancer.
Data from the phase 3 NRG-RTOG 0631 may inform future research assessing spinal radiosurgery in the oligometastatic setting, according to an expert from Stony Brook University Medical Center.

The addition of short-term androgen deprivation to dose-escalated radiation therapy did not yield a significant difference in quality-of-life outcomes vs radiotherapy alone for those with intermediate-risk prostate cancer, according to an expert from Henry Ford Health Cancer.

In addition to granting fast track designation to RRx-001, the FDA accepts an investigational new drug application for the agent to reduce and/or prevent radiation- and chemotherapy-associated oral mucositis.

An expert from Dana-Farber Cancer Institute indicates that urologists should refer patients with prostate cancer who present with multiple high-risk factors at surgery to a radiation and medical oncologist.

Fifteen-year results of the ProtecT prostate cancer trial may support the findings of the study’s 10-year follow-up data, according to an expert from Dana-Farber Cancer Institute.

The 10-year cumulative local recurrence rate in patients with early breast cancer receiving accelerated partial breast irradiation with multi-catheter brachytherapy does not significantly differ from those receiving whole-breast irradiation following breast-conserving surgery.

Increasing age, higher Gleason scores, and higher pathologic stages are predictors of mortality in patients with prostate cancer, according to an expert from Dana-Farber Cancer Institute.

Clinical trials highlight benefits, including radiographic progression-free survival following treatment with radioligand 177Lu-PSMA-617 in pretreated patients with metastatic castration-resistant prostate cancer.

Early data from ongoing clinical trials suggest the potential safety and efficacy of novel radium-223 combinations as treatment for metastatic castration-resistant prostate cancer.

Current clinical trials look to assess 177Lu-PSMA-617 in combination with other therapies including androgen deprivation therapy and docetaxel.

An expert from Dana-Farber Cancer Institute indicates that patients with prostate cancer who have 1 risk factor should undergo salvage radiotherapy following radical prostatectomy before their prostate-specific antigen level rises above 0.25 ng/ml.

An expert from Weill Cornell Medicine highlights key clinical data indicating the benefits of radium-223 in the treatment of patients with metastatic castration-resistant prostate cancer.

The risk of radionuclide exposure to the public reflects one reason urologists need to collaborate with radiation oncologists when administering radiopharmaceuticals to patients with prostate cancer.

Switching out beta emitters for alpha emitters, including radium-223, is one way to improve radiopharmaceutical treatment of prostate cancer, according to an expert from Weill Cornell Medicine.

Data from the phase 3 ROMAN trial and the phase 2b GT-201 trial support the new drug application for avasopasem in radiotherapy-induced severe oral mucositis for those with head and neck cancer.