
This review focuses on targeted therapies related to these pathways of interest for the treatment of advanced urothelial carcinoma, describing the evidence to support further investigation of these approaches.

Your AI-Trained Oncology Knowledge Connection!


This review focuses on targeted therapies related to these pathways of interest for the treatment of advanced urothelial carcinoma, describing the evidence to support further investigation of these approaches.

Current efforts utilizing genomic strategies to unravel the biology of urothelial carcinoma will undoubtedly lead to rational targets, new therapies, and a renewed enthusiasm among researchers and clinicians working in this field-which ultimately will improve the lives of patients with this devastating disease.

Advanced urothelial cancer remains, along with pancreatic cancer, one of the last solid tumors for which essentially no progress has been made for 25 years. It’s time to think out of the box, and to develop novel and creative ways of overcoming the real, but not insurmountable, logistical challenges to carrying out the needed clinical trials.

This review covers symptoms and complications in patients with late-stage pancreatic cancer, including venous thromboembolism, anorexia-cachexia, pain, and depression.
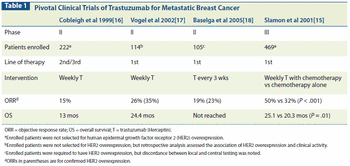
This article reviews clinical data informing the effective management of HER2-positive metastatic breast cancer, including the optimal sequence of HER2-targeted agents.
Additional insight into the biology of ER-positive breast cancers, particularly the higher risk luminal B cancers, could aid in identifying potential targets and new, effective therapies. And though the majority of triple-negative breast cancers are the “basal-like” subtype, significant proportions are in other subtypes.

If MGTs could predict which patients were most prone to late recurrence and thus might benefit from extended adjuvant endocrine therapy, it would be a huge advancement in the care and survivorship of our patients. More studies of MGTs are required to clarify their role in evaluating prognosis and predicting response to therapy in breast cancer.

Clinicians and bioinformatics experts must learn to speak the same language, starting with basic principles regarding analytical validity, clinical validity, and clinical utility. More than ever, a true partnership between clinical, laboratory, and bioinformatics scientists as part of a multidisciplinary team is needed to benefit our patients.

As more drugs become available in the HER2 arena, clinicians will be faced with increasing challenges regarding which sequences and combinations of drugs will be the best for their patients. In the era in which we practice, a great deal of this is likely to be dictated by the payers.

Optimal supportive care for patients with pancreatic cancer is essential. Putting these interventions into practice requires that oncologists and oncology teams incorporate innovations at both the individual and the system level.

FDA approval of palliative chemotherapy is largely based on disease-free and overall survival, quality of life, and symptom reduction; the latter should be routinely measured by the treating oncologist. Physician assessments of symptoms underreport symptom severity compared to patient-reported symptom assessments.

Third, how much do we really know about de novo and acquired resistance to trastuzumab and lapatinib? There are several possible clinical relevant mechanisms of trastuzumab resistance, including crosstalk with other receptors, amplification of the PI3K/AKT pathway, alteration of the trastuzumab binding domain, and loss of HER2 expression.

Last month brought the accelerated approval by the US Food and Drug Administration (FDA) of a fourth agent targeting the human epidermal growth factor receptor 2 (HER2) oncogene product: TDM-1 (Kadcyla), a conjugate of trastuzumab and a cytotoxic, emtansine.