
The FDA will review the supplemental biologics license application for alectinib based on data from the phase 3 ALINA study.

Your AI-Trained Oncology Knowledge Connection!


The FDA will review the supplemental biologics license application for alectinib based on data from the phase 3 ALINA study.

Patients with locally advanced or metastatic urothelial cancer and visceral disease may particularly benefit from enfortumab vedotin plus pembrolizumab, according to Amanda Nizam, MD.
Results from the phase 3 MOMENTUM trial led momelotinib to being approved by the European Commission for patients with myelofibrosis plus anemia and splenomegaly.

Data from the phase 2 DESTINY-PanTumor02 trial support the supplemental biologics license application for trastuzumab deruxtecan as a treatment for those with metastatic HER2-positive solid tumors.
Results from the randomized LASRE trial support the use of laparoscopic-assisted surgery for patients with low rectal cancer.

Therapies like yoga, music therapy, acupuncture, and natural health remedies have proven to be beneficial in mitigating anxiety and depression in adults with cancer, according to Linda E. Carlson, PhD, RPsych.

uMRD-negative status linked to recurrence-free outcomes with nadofaragene firadenovec in NMIBC

Findings from the phase 3 PROpel trial suggest that olaparib plus abiraterone acetate may help patients with metastatic castration-resistant prostate cancer live longer, according to Neal Shore, MD, FACS.
Treatment with belzutifan increased the time to disease progression, according to findings from the LITESPARK-005 trial.

Findings from the phase 3 CheckMate 67T trial showed noninferiority of subcutaneous nivolumab when compared with intravenous nivolumab.

Adjuvant pembrolizumab resulted in a reduction in the risk of death in patients with ccRCC.

Treatment with nivolumab did not reach the primary endpoint of disease-free survival when compared with placebo in patients with localized renal cell carcinoma at high risk of relapse after nephrectomy in the phase 3 CheckMate 914 trial.
Lenvatinib plus pembrolizumab showed a 70% probability of greater OS benefit among patients with advanced renal cell carcinoma.

Results from the phase 2 KEYNOTE-B61 trial showed prolonged survival in patients with advanced non–clear cell renal cell carcinoma.
Testing for HRR status prior to a diagnosis of metastatic castration-resistant prostate cancer may allow for earlier treatment and potentially greater clinical benefit with olaparib, according to Daniel J. George, MD.
Results from a post hoc sensitivity analysis of the phase 3 ARASENS trial appear to affirm the primary overall survival analysis findings.
Data from the GETUG-AFU 18 trial support high-dose radiation plus long-term androgen deprivation therapy as a standard of care in high-risk prostate cancer.
Supporting data for the EMA comes from the phase 3 EV-302 trial, which assessed the efficacy of enfortumab vedotin in combination with pembrolizumab compared with chemotherapy.

Four of 5 patients with prostate cancer and ctDNA positivity had disease progression following treatment with darolutamide plus androgen deprivation therapy in a phase 2 trial.

Six months of intensified androgen deprivation therapy plus next-generation anti-androgens may be an attractive option for those with prostate cancer and rising prostate specific antigen levels following prostatectomy.

The overall incidence of bone fractures with prior EBRT to the bone in patients with metastatic castration-resistant prostate cancer remains low.
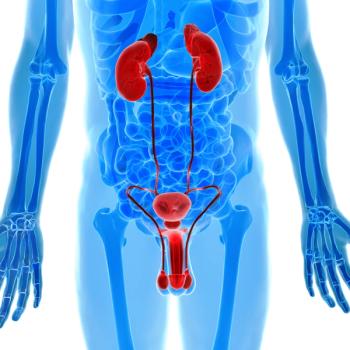
Additional education on tumor testing and PARP inhibitor therapy is warranted for patients with metastatic castration-resistant prostate cancer, according to Neal Shore, MD, FACS.

Findings support cabozantinib plus atezolizumab as a potential new treatment option for those with metastatic castration-resistant prostate cancer that has progressed on a novel hormonal therapy.

The FDA previously granted approval to frontline olaparib plus abiraterone/prednisone in May 2023 for BRCA1/2–, DDR–altered metastatic castration-resistant prostate cancer.

Darolutamide correlates with a marginally longer length of stay per hospitalization compared with placebo among those with metastatic hormone-sensitive prostate cancer.

Liver contrast-enhanced MRI resulted in a change in the local treatment plan for approximately a third of patients with colorectal liver metastases in the CAMINO study.

Treatment with pembrolizumab plus chemotherapy with or without bevacizumab yields an overall survival improvement regardless of squamous or nonsquamous cervical cancer histology.

High-grade adverse effects with zanidatamab plus palbociclib and fulvestrant seem to be uncommon in patients with HER2-positive, hormone receptor–positive, metastatic breast cancer, according to Sara Hurvitz, MD, FACP.

Patients with metastatic cancer who are receiving treatment at nonacademic or low-volume facilities appear more likely to receive end of life immunotherapy.

Expanding or updating coverage with Medicaid may lead to positive health outcomes among patients with non–small cell lung cancer following surgery, according to Leticia Nogueira, PhD, MPH.