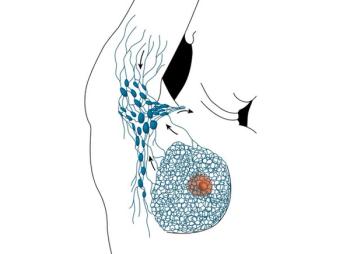
The use of a standard yearly physical examination to screen for thyroid cancer among high-risk survivors of childhood and young adult cancers resulted in a negative predictive value of 100% for clinically relevant thyroid cancer and a significant cost savings compared with regular ultrasound screening.