PFS and response rates were proved to be meaningful among patients receiving zolbetuximab plus mFOLFOX6 and nivolumab for metastatic gastric/GEJ cancer.

Your AI-Trained Oncology Knowledge Connection!


PFS and response rates were proved to be meaningful among patients receiving zolbetuximab plus mFOLFOX6 and nivolumab for metastatic gastric/GEJ cancer.

The overall survival benefit with the bemarituzumab combination in the phase 3 FORTITUDE-101 trial was consistent across key prespecified subgroups.

Data from the ROSELLA trial show a consistent benefit with relacorilant plus nab-paclitaxel across PROC subgroups.

T-DXd significantly improved iDFS compared with T-DM1 across various patient subgroups in high-risk, HER2-positive primary breast cancer with residual invasive disease.

Data from monarchE showed an OS benefit with abemaciclib-based therapy that was consistent across prespecified patient subgroups.

Updated findings from AURIGA support the value of adding subcutaneous daratumumab to lenalidomide maintenance in MRD-positive NDMM after transplant.

Belantamab mafodotin plus lenalidomide and dexamethasone yielded a stringent CR of 42.9% in patients with newly diagnosed multiple myeloma.

Iberdomide with daratumumab and dexamethasone led to an ORR of 93.1%, with stringent CRs in 30.1%, in those with newly diagnosed multiple myeloma.

Treatment with ISB 2001 led to an overall response rate of 74% across dose levels 1 to 9 in patients with relapsed/refractory multiple myeloma.

Additional time is necessary to confirm the depth and durability of responses observed in the phase 1 CAMMA 1 study.

The confirmed overall response rate with ABB-706 was 77% in patients with relapsed/refractory small cell lung cancer who received 2 prior lines of therapy.

David A. Braun, MD, PhD, discusses how neoantigen vaccines are redefining RCC treatment, with the potential to activate potent, lasting T-cell activity.

Frontline BNT327/PM8002 plus chemotherapy led to a confirmed ORR of 51.6% and a DCR of 90.3% in patients with unresectable pleural and peritoneal mesothelioma.

The KOMET-001 trial meets its primary end point of CR/CRh rate among patients with NPM1-mutated acute myeloid leukemia.

Multidisciplinary education on toxicities such as CRS and ICANS improves the safety and management of bispecific T-cell engager therapies in outpatient settings.

Results from the phase 2 DURBAC trial showed BVAC-C/durvalumab improved response in HPV+ cervical cancer.

Data from the ENVISION and ATLAS trials demonstrated clinically meaningful responses with UGN-102 in the treatment of low-grade, intermediate-risk NMIBC.

Subgroup analysis of the CALGB/SWOG 80702 trial investigated the impact of ctDNA status on DFS in stage III resected colon cancer patients treated with celecoxib or placebo.

Blinatumomab plus chemotherapy may represent a new treatment standard for most patients with standard-risk B-ALL, says Rachel E. Rau, MD.

Data support the addition of daratumumab to standard lenalidomide maintenance in patients with newly diagnosed multiple myeloma following transplant.

Data from the IMerge trial affirm the enduring responses and clinical benefit with imetelstat in those with transfusion-dependent MDS.
“In LITESPARK-005, PFS and response rates favored belzutifan vs everolimus across [several patient subgroups, including] IMDC risk, number of prior lines [of therapy], and number of prior VEGF TKIs, specifically,” said Laurence Albiges, MD, PhD.

Phase 1 data show no dose-limiting toxicities with sonrotoclax plus zanubrutinib for patients with relapsed/refractory CLL or SLL.
Progression-free survival and overall survival appear to be shorter in patients with TP53-mutant CLL vs those with TP53 wild-type disease.

High-grade cytokine release syndrome in the WU-CART-007 1001 trial was manageable with supportive care.
Findings may support reconsideration of guideline recommendations concerning the use of estrogen alone in postmenopausal individuals.

The phase 3 ALPINE and ASCEND studies were used in the MAIC analysis assessing zanubrutinib vs acalabrutinib in relapsed/refractory chronic lymphocytic leukemia.

The positive impacts on outcomes like recurrence-free survival with Orca-T highlight the importance of identifying that may benefit all key transplant outcomes, according to Alexandra Gomez Arteaga, MD.
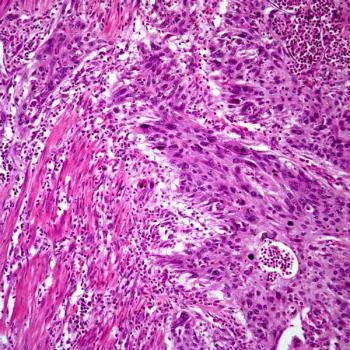
Interim findings from a phase 3 trial support adjuvant pembrolizumab as a new therapeutic option for those with muscle-invasive urothelial carcinoma at high risk of recurrence.
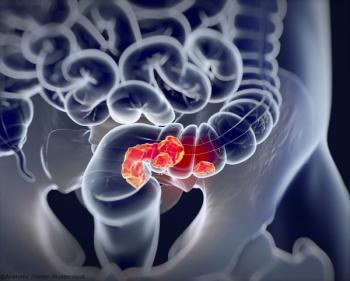
Neoadjuvant botensilimab plus balstilimab appears to be safe and effective in patients with colorectal cancer regardless of mismatch repair status in the phase 2 NEST-1 trial.
Published: February 18th 2023 | Updated:
Published: June 4th 2022 | Updated:
Published: December 11th 2022 | Updated:
Published: October 23rd 2023 | Updated:
Published: December 9th 2023 | Updated:
Published: December 14th 2022 | Updated: