The CAR T-cell therapy lisocabtagene maraleucel resulted in better efficacy versus standard of care with no new safety signals.

Your AI-Trained Oncology Knowledge Connection!


The CAR T-cell therapy lisocabtagene maraleucel resulted in better efficacy versus standard of care with no new safety signals.

A post hoc analysis of the CLEAR trial shows that patients may derive an overall survival benefit with lenvatinib plus everolimus versus sunitinib for frontline treatment of RCC, with efficacy ongoing.
Across 3 health-related quality of life scales, the combination of pembrolizumab plus lenvatinib had similar or better outcomes than sunitinib for patients with metastatic renal cell carcinoma receiving treatment in the frontline.
Two-year efficacy and safety data from a pivotal trial of nivolumab/ipilimumab plus chemotherapy as treatment of non–small cell lung cancer support the continued use of the combination.

A new treatment arm of a phase 2 trial examining PDS0101 plus pembrolizumab in patients with HPV-related head and neck cancers will be opened to patients with immune checkpoint inhibitor–pretreated disease.

Based on positive phase 3 results, a new drug application for sugemalimab to treat patients with locally advanced or unresectable stage III non–small cell lung cancer will likely be submitted to the FDA.
Based on data from a phase 1 trial, the FDA granted breakthrough therapy designation to teclistamab for certain patients with pretreated multiple myeloma.
Based on phase 3 data, plinabulin plus granulocyte colony-stimulating factor is being considered by the FDA for the prevention of chemotherapy-induced neutropenia.
Investigators successfully established the phase 2 dose of APVO436 for patients with acute myeloid leukemia or myelodysplastic syndrome after an acceptable safety profile was achieved in patients with responses to therapy.

Objective response data from a phase 2 trial supported the FDA’s decision to grant infigratinib accelerated approval to treat patients with certain a certain type of cholangiocarcinoma.

Based on data from the phase 1/2 CodeBreaK 100 trial, the FDA approved the KRAS G12C inhibitor sotorasib.
Based on data from the CARTITUDE-1, the BCMA-targeting CAR T-cell therapy ciltacabtagene autoleucel moves forward towards regulatory approval in multiple myeloma.

The ready-to-use subcutaneous formulation of leuprolide mesylate, Camcevi, was approved for use in patients with prostate cancer by the FDA.

Investigators aimed to determine if oncologic outcomes of patients in the real-world setting matched those of a pivotal clinical trial that led to the approval of eribulin mesylate in patients with metastatic breast cancer.

The 2020 Trending Now in Cancer report highlighted a shift in oncology care resulting from the COVID-19 pandemic.
Based on phase 3 data supporting the use of ublituximab in combination with umbralisib versus an existing standard-of-care regimen for chronic lymphocytic leukemia and small cell lymphoma, the FDA has moved forward with a review of the application for approval.
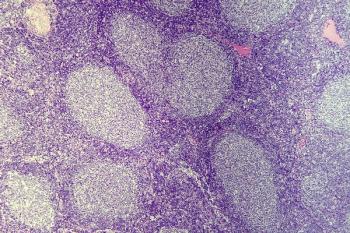
The radioimmunotherapy 177-Lu lilotomab satetraxetan in combination with rituximab led to a 100% response rate in a small cohort of patients with follicular lymphoma who were receiving treatment in the second-line setting.
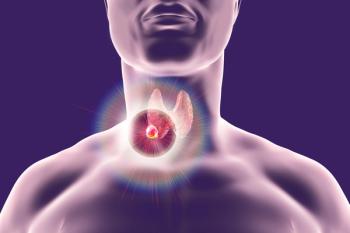
An affinity-tuned CAR T-cell product will be evaluated in thyroid cancer with a fast track designation from the FDA.

A RAF/MEK plus FAK inhibitor combination will be given an expedited review by the FDA as therapy for patients with recurrent low-grade serous ovarian cancer.

Promising results from a phase 1b trial of cabozantinib in combination with atezolizumab for patients with high-risk, locally advanced or metastatic castration-resistant prostate cancer are expected to lead to regulatory submission.
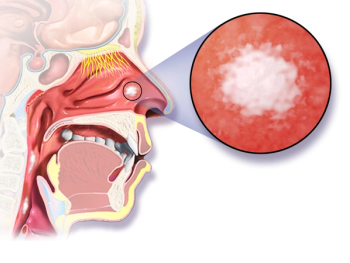
Penpulimab may be a future treatment option for patients with nasopharyngeal cancer in the third line of therapy dependent on success of a recent biologics license application submitted to the FDA.

Black women with triple-negative breast cancer may have worse survival outcomes compared with White women, even after adjusting for external factors.

The first targeted therapy for EGFR exon 20 insertion mutation–positive non–small cell lung cancer is now available for patients in the United States.
Phase 3 data supported the FDA in their decision to grant priority review to a new drug application for maribavir as therapy for certain patients with cytomegalovirus following transplant.

Adjuvant nivolumab for resected esophageal or gastroesophageal junction cancer was granted full approval by the FDA based on statistically significant improvements in disease-free survival over placebo in a phase 3 trial.
Prostate cancer in African American men was improved with higher rates of screening for prostate-specific antigen.
Seventeen years of data indicate that HPV-related cervical cancer incidence is declining, but other tumors related to the virus persist.

Based on phase 1 and 2 trial results and pooled safety data from several clinical trials, zanubrutinib moves forward with a priority review in relapsed/refractory marginal zone lymphoma.

Based on results of a phase 3 trial demonstrating the superiority of sintilimab versus placebo plus chemotherapy for nonsquamous non–small cell lung cancer, the FDA considers approval of the PD-1 inhibitor injection.
Recommendations published in JAMA are set to change colorectal cancer screening guidance for all individuals in the United States.