Articles by Michael Kaufman

Leaders of the American Association for Cancer Research (AACR) have expressed deep concern that the ability of cancer researchers to bring the promise of science to improve outcomes for cancer patients in the United States is in peril due to a decade of declining budgets at the National Institutes of Health (NIH).
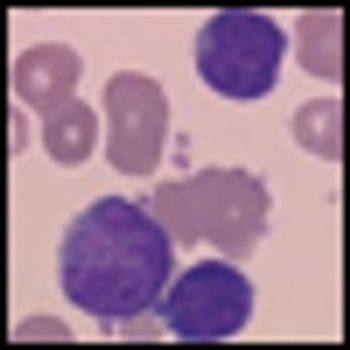
Inotuzumab ozogamicin has achieved a markedly long antitumor response in patients with refractory or relapsed indolent B-cell non-Hodgkin lymphoma (NHL) in an ongoing phase II study. Interim findings were reported by lead investigator Kenneth Luu, PhD, associate director of Pfizer global R&D.

Photoacoustic tomography (PAT), a new imaging technique that relies on light and sound, provides in vivo multiscale nonionizing functional and molecular imaging without the radiation emitted by x-rays and CT scans, explained Lihong V. Wang, PhD, who led the team of developers at Washington University in St. Louis.
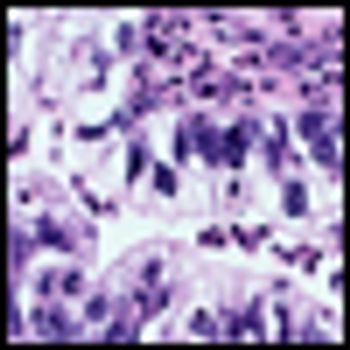
Addition of a Janus kinase 2 (JAK2) inhibitor to therapies that target commonly altered oncogenes such as KRAS or EGFR could improve clinical outcomes for patients with non–small cell-lung cancer.

Selumetinib, a small-molecule MEK inhibitor, controlled recurrent low-grade serous ovarian cancer in 81% of patients treated. Current first-line defense against this disease (surgery followed by cytotoxic chemotherapy) has met with limited success.

Development of a novel method for drug discovery using active compounds as pharmacodynamic biomarkers in triple-negative breast cancer cell lines.
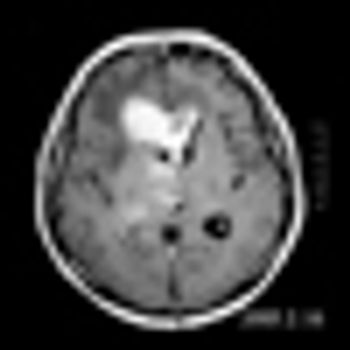
Hypofractionated stereotactic radiotherapy (SRT) appears to be safe and effective in preventing recurrence at resection cavities following surgical resection of brain metastasis and may spare many patients from whole brain radiotherapy (WBRT) and its adverse effects.
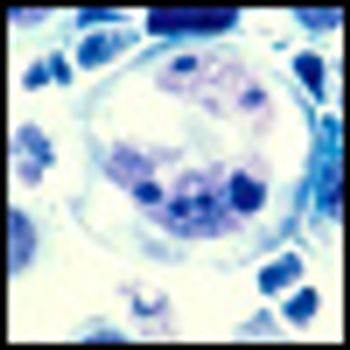
A combination of TL32711, an investigational second mitochondrial-derived activator of caspases (Smac), and tumor necrosis factor-related apoptosis inducing ligand at low concentrations produced marked apoptosis in germinal center lines, with minimal to no effect for each agent alone.

Treatment with tremelimumab stabilized patients with advanced hepatocellular carcinoma due to chronic hepatitis C infection for more than 12 months, according to data from a phase II clinical trial presented at the AACR annual meeting.
Preliminary findings of a phase I/II randomized clinical trial indicate that SGI-110, a novel DNA methylation inhibitor, is safe, well tolerated and efficacious in patients with acute myelogenous leukemia.

Use of neoadjuvant metformin prior to radical prostatectomy helped to reduce metabolic effects and slow the growth rate of cancer in a single-center phase II study conducted among men with confirmed prostate cancer.
The antiangiogenic agent pazopanib demonstrated clinically meaningful activity in patients with refractory urothelial cancer in a phase II proof-of-concept study, identifying pazopanib as the first targeted compound to have clinically meaningful activity in patients with refractory urothelial cancer.
A combination of cixutumumab, a type 1 insulin-like growth factor receptor inhibitor, and temsirolimus, a mammalian target of rapamycin (mTOR) inhibitor, showed evidence of activity in refractory Ewing’s sarcoma tumors as well as small-round-cell tumors in a phase I multicenter clinical study.
Updated findings from a multicenter phase Ib/II clinical trial suggest that the novel Bruton’s tyrosine kinase (BTK) inhibitor PCI-32765 may be an important new targeted treatment approach for patients with chronic lymphocytic leukemia.

Romiplostim, a synthetic protein that binds to and stimulates the thrombopoietin receptor, induced a rapid platelet response in adult patients with primary immune thrombocytopenia (ITP) and maintained a consistent safety profile in an international phase III trial.
Obinutuzumab (GA101) achieved higher response rates than rituximab in the first head-to-head trial of the two biologic agents in patients with relapsed non-Hodgkin lymphoma.
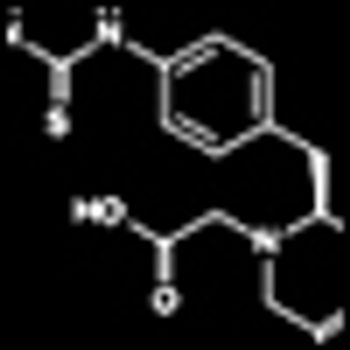
Encouraging findings were reported from multiple studies of ibritumomab tiuxetan (Zevalin), a CD20-directed radiotherapeutic antibody, in diverse patient groups with follicular lymphoma.

Bevacizumab (Avastin) improved progression-free survival (PFS) in women with HER2-positive locally recurrent or metastatic breast cancer by an average of 3 months when added to standard treatment as first-line therapy in the multinational, randomized, phase III AVEREL study.
Results of 4 trials involving bisphosphonates in a range of protocols and patient cohorts suggest that the role of these agents in preventing recurrence of breast cancer remains to be defined. In 2 of the 4 studies reported, favorable outcomes were obtained following intravenous administration of zoledronic acid. Neither of two trials in which a bisphosphonate was administered orally, however, achieved its primary endpoint.
Although some preventive steps can now be taken by women to reduce environmental factors that contribute to breast cancer risk, much more research is needed to clarify the role of recognized and suspected environmental factors, according to a new report issued by the Institute of Medicine.
Updated findings from the pivotal phase III Breast Cancer Trials of Oral Everolimus (BOLERO-2) study confirm dramatic improvement in progression-free survival (PFS) in women with metastatic breast cancer when the immunosuppressant agent is combined with the hormonal therapy exemestane.
Addition of pertuzumab to a standard chemotherapy combination of trastuzumab and docetaxel led to a 38% reduction in risk of disease worsening or death in patients with HER2-positive metastatic breast cancer, reported investigators from the randomized, double-blind, placebo-controlled phase III CLEOPATRA study.























