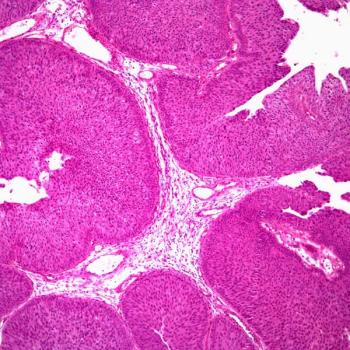
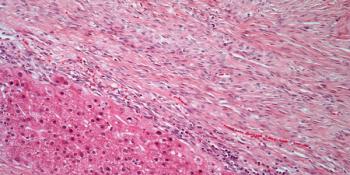
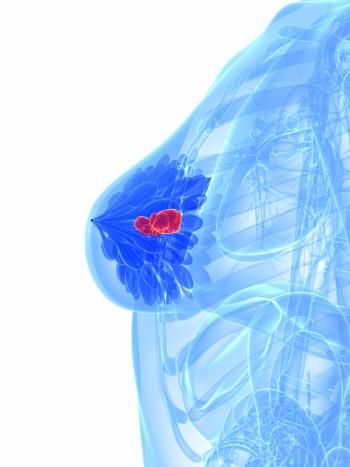
Rebecca M. Shulman, MD, and Zachary Kiss, DO, discuss findings from a study evaluating differences in outcomes with radiotherapy and disease characteristics of patients with breast cancer harboring BRCA mutations compared with those without mutated disease.