Avyakta Kallam, MBBS, gave a look as to why she decided to compile a comprehensive review in mantle cell lymphoma.

Your AI-Trained Oncology Knowledge Connection!


Avyakta Kallam, MBBS, gave a look as to why she decided to compile a comprehensive review in mantle cell lymphoma.
Combination treatment with cabozantinib and atezolizumab did not produce any new safety signals in the treatment of those with metastatic castration-resistant prostate cancer in the phase 3 CONTACT-02 trial.
Investigators of a cohort study report that the withdrawal rate from clinical trials in oncology appear to be higher among Hispanic patients and across placebo-controlled trials, but lower in trials with radiation use as part of therapy.
The FDA allows for expanded bridging therapies in addition to other trial protocol amendments to enable investigators to continue their evaluation of CART-ddBCMA in relapsed/refractory multiple myeloma.

Saad. Z. Usmani, MD, leads a panel of experts in discussing toxicities associated with treatment for multiple myeloma.
During Morning Rounds, Petros Grivas, MD, and his treating team debate the diagnosis of a patient with urothelial carcinoma as it has an unusual presentation.
A larger, phase 2 trial is planned to further evaluate pembrolizumab plus doxorubicin, vinblastine, and dacarbazine in patients with newly diagnosed Hodgkin lymphoma.

Current treatment options for patients with EGFR exon 20 non–small cell lung cancer were discussed among a panel of experts in a recent Frontline Forum.
Investigators will work with the FDA to assess the root cause of a grade 5 serious adverse effect in phase 2 PLAT-08 trial evaluating SC-DARIC33 in pediatric acute myeloid leukemia.
Phase 2 data support administering trastuzumab in combination with ramucirumab and paclitaxel for patients with HER2-positive gastric or gastroesophageal junction cancer.

The HLA-LOH test makes use of data produced with the xT CDx assay to identify patients with tumors experiencing allele-specific loss of heterozygosity who may benefit from specific targeted treatments.
Data indicate that it may be safe to pause endocrine therapy for patients to attempt to conceive, and experts in the space review how it has impacted treatment strategies.

The FDA companion diagnostic designation for FoundationOne CDx may improve access to treatment with niraparib/abiraterone acetate dual action tablets in those with metastatic castration-resistant prostate cancer harboring a BRCA mutation.
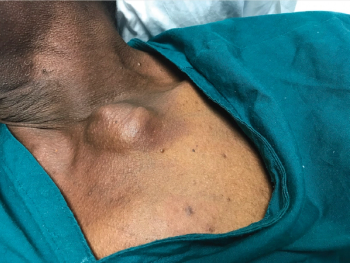
Rohit Gupta, MD, et al review a case study of a 70-year-old man who presented with a head mass, and the final diagnosis was hepatocellular carcinoma.

Findings from a phase 1 study highlight that ivosidenib tablets did not raise any new safety signals in the treatment of patients with IDH1-mutated relapsed/refractory myelodysplastic syndromes.
Investigators of the phase 1/2 Acclaim-3 trial will assess quaratusugene ozeplasmid in combination with atezolizumab as a treatment for those with extensive-stage small cell lung cancer.
Avyakta Kallam, MBBS and Julie Vose MD, MBA, review available treatment options for patients with mantle cell lymphoma.

Future developments in the sarcoma space may also involve research on circulating tumor DNA and metabolic therapies, according to Brian Van Tine, MD, PhD.
Findings from a real-world study suggest the importance of novel agents for improving efficacy outcomes and tolerability in elderly patients with mantle cell lymphoma.
In the August Letter to Readers, Julie M. Vose, MD, MBA, discusses the recent cisplatin shortages and how this can be mitigated in the future.

Physician burnout is not just a phase, but rather a mental health issue that has made itself well-known in the medical community, with many clinicians trying to find strategies to cope with it.

Supporting data for the FDA approval of melphalan administered via percutaneous hepatic perfusion for metastatic uveal melanoma come from the phase 3 FOCUS study.

Elranatamab-bcmm is now approved for patients with relapsed/refractory multiple myeloma who have received at least 4 prior lines of therapy.
Results from the phase 3 MOMENTUM trial support the use of momelotinib as a treatment for patients with myelofibrosis, including those with anemia or thrombocytopenia.
Cutting out radiotherapy appears to be safe while improving quality of life in patients with POLE-mutated endometrial cancer.
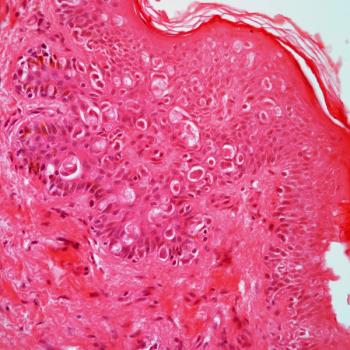
Treatment with immune checkpoint inhibitors does not raise any new safety signals among patients with locally advanced or metastatic penile squamous cell carcinoma in an international cohort study.

Experts discuss key data updates in real-world newly diagnosed multiple myeloma practices, and how these findings may change the treatment paradigm.

Guidelines from the Society of Gynecologic Oncology may help with managing the ongoing chemotherapy shortage in the treatment of patients with gynecologic cancers, according to Brian Slomovitz, MD, MS, FACOG.
Investigators also report that disease-free survival was not associated with regional node irradiation treatment in favorable-risk, node-positive breast cancer.
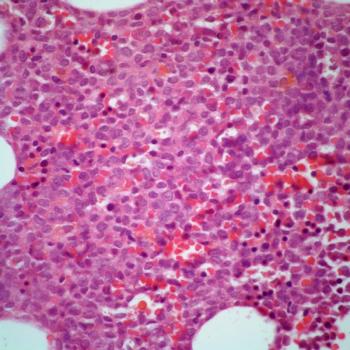
The experimental agent appears tolerable in patients with newly diagnosed FLT3-mutated acute myeloid leukemia in a phase 1b trial.