
Dostarlimab plus chemotherapy produces notable benefits among patients with advanced, mismatch repair deficient endometrial cancer in the phase 3 RUBY trial.

Your AI-Trained Oncology Knowledge Connection!


Dostarlimab plus chemotherapy produces notable benefits among patients with advanced, mismatch repair deficient endometrial cancer in the phase 3 RUBY trial.

Investigators note that although therapy-related myelodysplastic syndromes or acute myeloid leukemia are not common, efforts to reduce treatment-associated toxicity in survivors of lymphoid neoplasms are needed given the poor prognosis associated with the diagnosis.

Treatment with batiraxcept plus paclitaxel did not produce any new safety signals among patients with platinum-resistant ovarian cancer in the phase 3 AXLerate-OC trial.

CAR T-cell therapies and immunotherapy agents may offer up new options and even become standard of care in certain sarcoma subtypes.

Investigators of a retrospective study report that an AI deep learning model may also predict negative margin probabilities better than conventional modalities.
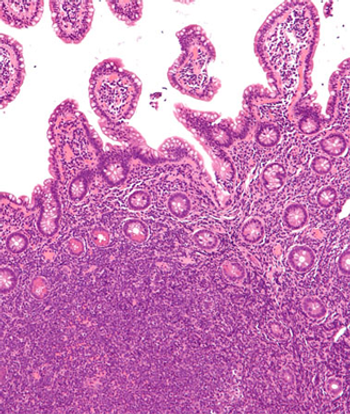
Levels of soluble CD163 appear to be higher in those with p53 abnormalities, elevated lactate dehydrogenase, and low hemoglobin in a study cohort of patients with mantle cell lymphoma.

Results from the phase 3 SUNLIGHT trial support the approval of trifluridine/tipiracil plus bevacizumab in patients with previously treated, metastatic colorectal cancer.

Elraglusib is currently under evaluation as a treatment for patients with several types of advanced cancers as part of the phase 1/2 1801 trial.

Investigators of a meta-analysis report no significant difference in outcomes when comparing trimodality therapy and radical cystectomy for the treatment of muscle-invasive bladder cancer.

There are several novel treatments that may be beneficial in several sarcoma subtypes including CAR T-cell therapies and immune checkpoint inhibitors, according to Sandra P. D’Angelo, MD.

Brian Van Tine, MD, PhD, speaks about several agents and combination regimens that are currently under investigation in the sarcoma space, and potential next steps in research including immunotherapies and vaccine-based treatments.

The fourth-generation tyrosine kinase inhibitor yields several partial responses and a tolerable safety profile in a small population of patients with EGFR-mutated non–small cell lung cancer in a phase 1 trial.

The investigational T-cell therapy IVS-3001 is currently under evaluation as part of a phase 1/2 trial in patients with advanced or metastatic solid tumors that are HLA-G positive.

Recommendations penned by the American Society of Clinical Oncology and Society for Gynecologic Oncology may be critical in managing the ongoing chemotherapy shortage, according to Michael Ganio, PharmD, MS, BCPS, FASHP.
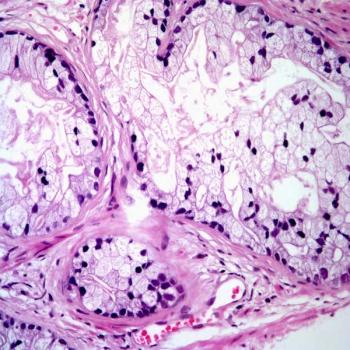
Incorporating PSMA PET into pre-surgical risk assessment may help urologists determine whether surgery should be performed on patients with advanced prostate cancer, according to Loïc Djaïleb, MD, PhD.

Findings from the phase 3 RUBY trial support the FDA’s approval of dostarlimab/chemotherapy as a treatment for advanced or recurrent endometrial cancer that is either mismatch repair deficient or microsatellite instability–high.
The approval from the European Commission is based on findings from the phase 3 TROPiCS-02 study.

The regulatory agency requests enhanced product testing and additional controls in their market application review of denileukin diftitox for the treatment of those with relapsed/refractory cutaneous T-cell lymphoma.
Investigators of a phase 1 study evaluating cosibelimab in patients with locally advanced or metastatic cutaneous squamous cell carcinoma plan to share updated results at a future medical conference.

Experts review current treatments for metastatic castration-sensitive prostate cancer, centering their discussion around a patient case.

Chemotherapy agents imported from Chinese manufacturers may have barcodes that don’t work or be missing an NDC number, according to Michael Ganio, PharmD, MS, BCPS, FASHP.
Experts in the multiple myeloma space discuss recent developments and how to implement new practices in real-world settings.
“I’d love to see us figure out a way to optimize treatment and personalize care. Biomarkers will help us get there, but we also need to be bold in our clinical trial designs.”

The potential reduction in relapse in patients with unresectable chronic lymphocytic leukemia appears to happen regardless of minimal residual disease status 3 months post-treatment and immunoglobulin heavy-chain variable region mutational status.
Co-editor-in-Chief Howard S. Hochster, MD, writes about the past and present of the phase 2/3 PROSPECT trial.

Treatment with neoadjuvant pembrolizumab among those with estrogen receptor–positive, HER2-negative breast cancer in the phase 3 KEYNOTE-756 trial does not produce any new safety signals.
Brian Van Tine, MD, PhD, discusses clinical trials assessing several regimens that may lead to novel treatment options for those with desmoid tumors, dedifferentiated liposarcoma, and other hard-to-treat sarcoma subtypes.

Results of a survey from the National Comprehensive Cancer Network indicate that almost all responding institutions are impacted by the carboplatin shortage.

Experts in the genitourinary and gynecology fields discuss how they have adapted and adjusted treatment for their patients amid the United States’ shortage of cisplatin and carboplatin.

The safety profile of trastuzumab deruxtecan among patients with HER2-expressing solid tumors in the phase 2 DESTINY-PanTumor02 trial appears to be comparable with previous reports of the agent.