In recognition of Kidney Cancer Awareness Month, CancerNetwork® spoke with Laurence Albigès, MD, PhD, about immunotherapy combination trials for renal cell carcinoma that read out at the 2022 Genitourinary Cancers Symposium.

Your AI-Trained Oncology Knowledge Connection!


In recognition of Kidney Cancer Awareness Month, CancerNetwork® spoke with Laurence Albigès, MD, PhD, about immunotherapy combination trials for renal cell carcinoma that read out at the 2022 Genitourinary Cancers Symposium.
Primo Nery Lara, Jr., MD, talks treatment of metastatic renal cell carcinoma in the first-line setting at the 15th Annual Interdisciplinary Prostate Cancer Congress® and Other Genitourinary Malignancies, hosted by Physicians’ Education Resource®, LLC.

At the 15th Annual Interdisciplinary Prostate Cancer Congress® and Other Genitourinary Malignancies, Daniel P. Petrylak, MD, spoke about how immunotherapy has influenced treatment for patients with bladder cancer.

Jun Gong, MD, spoke with CancerNetwork® about the latest research from the journal ONCOLOGY® on elderly patients with muscle-invasive bladder cancer.

Shilong Wu, MD, and co-authors discuss how patients with thymoma are affected by prior cancer history.

Yelena Y. Janjigian, MD, spoke about using immunotherapy across settings to treat localized gastric cancer.
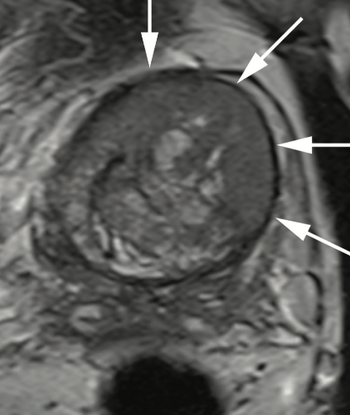
Dr. Judd W. Moul, MD, and colleagues present the case of a man, aged 73 years, with a prostate-specific antigen level of 110 ng/mL after 4 negative prostate biopsies and 4 negative prostate MRIs.

Daniel P. Petrylak, MD, reviews the current systemic therapy treatment paradigm in metastatic urothelial carcinoma.
The BRACAnalysis CDx test received FDA approval as a companion diagnostic for olaparib for the treatment of patients with germline BRCA-mutated HER2 negative high-risk early-stage breast cancer.

Axillary ultrasound and biopsy appear to be an effective strategy to identify nodal disease in patients with early-stage triple-negative breast cancer.
Axel Merseburger, MD, PhD, looked back on 2022 ASCO GU and the new data presented for the treatment of prostate cancer.

In an interview with CancerNetwork®, Eran Ben-Ayre, MD, spoke about guidelines for clinicians treating patients by way of telehealth during the pandemic, and how they can be adapted for future practices.
A phase 1 study of davoceticept and pembrolizumab for advanced malignancies received a partial clinical hold from the FDA following a patient death.
In an interview with ONCOLOGY®, Evan B. Bryson, PharmD, BCOP, offers a comprehensive review of real-world treatment considerations of amivantamab as therapy for patients with non–small cell lung cancer.

The FDA approval of olaparib comes from the results of the phase 3 OlympiA trial that tested the treatment vs a placebo.

At 2022 ASCO GU, Daniel M. Geynisman, MD, spoke with CancerNetwork® about his strategy for treating renal cell carcinoma across lines of therapy.

“Team science is important for being open-minded [and] thinking outside of the box.”

Pediatric patients with newly diagnosed pediatric T-cell lymphoblastic lymphoma experienced an improvement in survival following treatment with bortezomib and chemotherapy.

Adverse effects associated with treatment with olaparib that led to dose reductions and interruptions did not impact survival benefit for patients with platinum-sensitive ovarian cancer.

Laparoscopy compared with open gastrectomy was found to produce better overall survival outcomes at the 5-year follow-up analysis for patients with advanced gastric cancer.

Treatment with sacituzumab govitecan-hziy in patients with hormone receptor–positive/HER2-negative metastatic breast cancer who previously received treatment with endocrine therapy, CDK4/6 inhibitors, and 2 to 4 lines of chemotherapy resulted in a statistically significant improvement in progression-free survival vs physician’s choice of chemotherapy.

For Kidney Cancer Awareness Month, CancerNetwork® spoke with Chung-Han Lee, MD, PhD, about research in renal cell carcinoma that has the greatest potential to impact the standard of care.

An analysis presented at 2022 IGCC showed that the level of CD8 + cells in the outer margin of gastric cancer tumors was significantly associated with survival outcomes and could inform prognosis.

Applications for darolutamide were submitted to the FDA and European Medicines Agency for the treatment of patients with non-metastatic hormone-sensitive prostate cancer.

The FDA granted fast track designation to the BCMA-targeting Tri-specific T-cell Activating Construct, HPN217, for patients with relapsed or refractory multiple myeloma who received at least 4 lines of prior therapy.
Men with MSI-high gastric cancer may have shorter overall survival than women, regardless of treatment modality.

Chung-Han Lee, MD, PhD, spoke about necessary research needed moving forward for patients with metastatic renal cell carcinoma.
Kohie Shitara, MD, spoke about the use of immunotherapy in the first-line setting for metastatic gastric cancer.

The phase KEYNOTE-716 trial, which assessed the use of adjuvant pembrolizumab in patients with resected stage IIB and IIC melanoma, met its secondary end point of distant metastasis-free survival.

Post-resection survival could be predicted by FAM46C expression in patients with gastric adenocarcinoma.