Recently announced phase 3 data show similar progression-free survival results between melflufen and pomalidomide, the most used medicine for patients with relapsed or refractory multiple myeloma.

Your AI-Trained Oncology Knowledge Connection!


Recently announced phase 3 data show similar progression-free survival results between melflufen and pomalidomide, the most used medicine for patients with relapsed or refractory multiple myeloma.
An affinity-tuned CAR T-cell product will be evaluated in thyroid cancer with a fast track designation from the FDA.
A RAF/MEK plus FAK inhibitor combination will be given an expedited review by the FDA as therapy for patients with recurrent low-grade serous ovarian cancer.
Promising results from a phase 1b trial of cabozantinib in combination with atezolizumab for patients with high-risk, locally advanced or metastatic castration-resistant prostate cancer are expected to lead to regulatory submission.

CancerNetwork®’s podcast dives into an article focused on treatment options for older, transplant-ineligible patients with multiple myeloma.

Look back at some of the important news and notes from last week you might have missed in the world of oncology. The FDA granted priority review to zanubrutinib, while 2 features from the journal ONCOLOGY® published on belantamab mafodotin and renal cell carcinoma, respectively, were popular with readers.
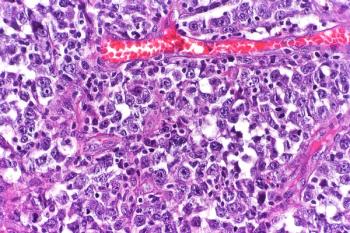
Phase 2 data supporting the use of loncastuximab tesirine in patients with diffuse large B-cell lymphoma published in The Lancet Oncology show the agent inducing a response in about half of patients with pretreated disease.
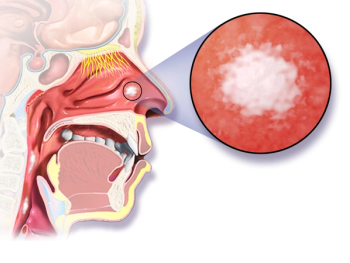
Penpulimab may be a future treatment option for patients with nasopharyngeal cancer in the third line of therapy dependent on success of a recent biologics license application submitted to the FDA.
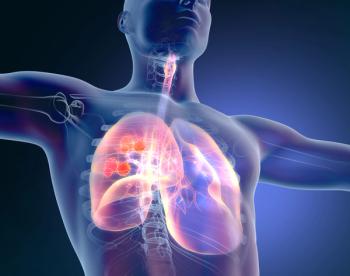
Solid nodules, peritumoral interstitial thickening, and pleural contact can play a role in carefully selecting optimal patients to undergo sublobar resection instead of more extensive surgery.
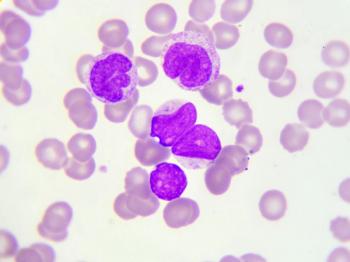
The interim activity and safety data observed were positive for both FT516 and FT538 monotherapy for patients with relapsed or refractory acute myeloid leukemia.
Black women with triple-negative breast cancer may have worse survival outcomes compared with White women, even after adjusting for external factors.

CancerNetwork® spoke with Cynthia X. Ma, MD, PhD, during the American Association for Cancer Research Annual Meeting 2021 about data supporting the potential use of the exemestane/leuprolide acetate plus pembrolizumab combination.
Data extrapolated from healthy lifestyle scores and polygenic risk scores found that healthy lifestyle changes were associated with a greater reduction in the risk in developing colorectal cancer for patients with a high genetic risk of disease development.

BeiGene’s press release details the positive progression-free survival data observed with the combination of tislelizumab plus chemotherapy compared with placebo plus chemotherapy for patients with recurrent or metastatic nasopharyngeal cancer.

The first targeted therapy for EGFR exon 20 insertion mutation–positive non–small cell lung cancer is now available for patients in the United States.
Phase 3 data supported the FDA in their decision to grant priority review to a new drug application for maribavir as therapy for certain patients with cytomegalovirus following transplant.
Results of a study found that ctDNA could provide an immediate benefit for mitigating selection bias in DLBCL-centered clinical trials.

In this special edition of the “Oncology Peer Review On-The-Go” podcast, CancerNetwork spoke with Reshma L. Mahtani, DO, about genomic testing to optimize treatment outcomes in HR-positive breast cancer.

Adjuvant nivolumab for resected esophageal or gastroesophageal junction cancer was granted full approval by the FDA based on statistically significant improvements in disease-free survival over placebo in a phase 3 trial.
Based on outcomes from a study involving patients with cancer, Medicaid may benefit from expansion in certain areas where eligibility limits hinder adequate coverage.
Prostate cancer in African American men was improved with higher rates of screening for prostate-specific antigen.
Seventeen years of data indicate that HPV-related cervical cancer incidence is declining, but other tumors related to the virus persist.
Findings from the phase 3 IMpower010 trial support the use of atezolizumab as adjuvant therapy for patients with stage II to IIIA non–small cell lung cancer.
Progression-free survival more than doubled when relatlimab was added to nivolumab to treated patients with previously untreated, unresectable or metastatic melanoma.

The combination of alpelisib and fulvestrant maintained a tolerable safety profile for patients with PIK3CA-mutated, hormone receptor–positive, HER2-negative advanced breast cancer who progressed after previous treatment with a CDK4/6 inhibitor.

Based on phase 1 and 2 trial results and pooled safety data from several clinical trials, zanubrutinib moves forward with a priority review in relapsed/refractory marginal zone lymphoma.

Alessi detailed his research into cancer aneuploidy and its predictive capabilities for treating patients with non–small cell lung cancer.

In this special edition of the “Oncology Peer Review On-The-Go” podcast, CancerNetwork spoke with Vijayakrishna (VK) Gadi, MD, PhD about the current role of genomic testing in HR-positive breast cancer.
Based on results of a phase 3 trial demonstrating the superiority of sintilimab versus placebo plus chemotherapy for nonsquamous non–small cell lung cancer, the FDA considers approval of the PD-1 inhibitor injection.
Recommendations published in JAMA are set to change colorectal cancer screening guidance for all individuals in the United States.