
Merck, the manufacturer of pembrolizumab, announced that it would be withdrawing the agent’s indication for the treatment of patients with previously treated SCLC.

Your AI-Trained Oncology Knowledge Connection!


Merck, the manufacturer of pembrolizumab, announced that it would be withdrawing the agent’s indication for the treatment of patients with previously treated SCLC.

Schilsky joined CancerNetwork®’s podcast to discuss ASCO’s Advance of the Year, the top priorities moving forward, and the COVID-19 pandemic.

An analysis revealed that as a third- or fourth-line treatment for advanced renal cell carcinoma, tivozanib significantly increased Q-TWiST compared with sorafenib, primarily through an increase in TWiST.

The developers of an oral formulation of paclitaxel will have to revisit efficacy and safety data in order to provide sufficient evidence for FDA approval.
Data published in The Lancet found that the PD-L1 inhibitor cemiplimab improved overall and progression-free survival for patients with advanced non–small cell lung cancer with PD-L1 of at least 50%.

Based on phase 2 data, the FDA granted accelerated approval to melphalan flufenamide in combination with dexamethasone for the treatment of multiple myeloma following 4 or more prior lines of therapy.
“Emerging evidence supports a possible role for overall dietary patterns that, in totality, emphasize habitually consuming fruits, vegetables, grains, and low-fat dairy and reducing red meat and alcohol intake,” wrote the study authors.
The medical oncologist at Memorial Sloan Kettering Cancer Center discussed the significance of the phase 3 CheckMate 9ER study results.

Data from the Journal of the American College of Surgeons suggest patients 70 years or older with esophageal or esophagogastric junction cancers should be evaluated for optimal curative therapy.
The first single shot COVID-19 vaccine, and the third overall, to receive Emergency Use Authorization will start shipping to all parts of the United States immediately following a meeting of the FDA’s Vaccines and Related Biological Products Advisory Committee.

A study published in JAMA Oncology found that, of 6 tested systemic treatment options, abiraterone acetate and apalutamide were the likeliest to improve overall survival when combined with ADT for patients with metastatic castration-sensitive prostate cancer.
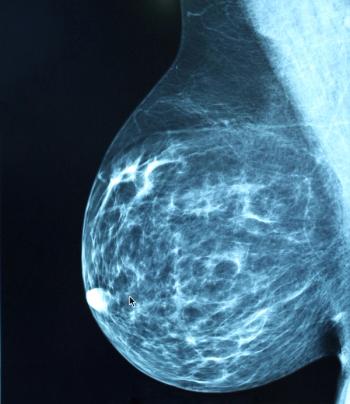
Given these study findings, investigators suggested that future efforts should address racial/ethnic, educational, financial, and geographic barriers to receiving digital breast tomosynthesis screening at the facility level.

An analysis revealed that as a third- or fourth-line treatment for RCC, tivozanib significantly increased Q-TWiST compared with sorafenib, primarily through an increase in TWiST.

The KEYNOTE-010 study evaluated the use of either pembrolizumab or docetaxel in patients with previously treated, PD-L1–positive advanced non–small cell lung cancer.

“These updated results from the phase 2 MERECA trial underscore the positive impact on overall survival that ilixadencel may achieve for [patients with kidney cancer],” said Sven Rohmann, MD, PhD.

Study results from the Journal of Medical Internet Research indicated that medication adherence by way of reminders on mobile devices for patients with acute lymphoblastic leukemia and their caregivers may have validity.

Data published in The Lancet Oncology investigating patients with malignant pleural mesothelioma determined that surgery for mesothelioma after radiotherapy, or SMART, can produce positive early- and long-term effects.
Giorgio Trinchieri, MD, of the National Cancer Institute’s Center for Cancer Research joined CancerNetwork® to discuss enhancing the gut microbiome by way of fecal transplant for better immunotherapy responses.

Data published in The Lancet Oncology found that a hypofractionated radiation schedule of 55 Gy in 20 fractions is noninferior to a schedule of 64 Gy in 32 fractions for patients with this disease.
According to investigators, study findings could help guide treatment decisions and future research, as well as suggest new biomarkers for exploration.

Data examining 4 low-value breast cancer procedures found an association between facility-level characteristics and the use of these procedures, suggesting a need for de-implementation targeting efforts in various facilities.

Results of a phase 2 trial testing the efficacy of idecabtagene vicleucel in patients with heavily pretreated myeloma are reported.
In the phase 3 CheckMate 9ER trial, investigators found that nivolumab plus cabozantinib demonstrated improved efficacy and prolonged survival among patients with previously untreated advanced renal cell carcinoma.
In the CheckMate 9ER trial, investigators compared combination treatment with nivolumab and cabozantinib versus sunitinib in patients with previously treated advanced renal cell carcinoma.

The PHERGain II trial will investigate the treatment of patients with early HER2-positive breast cancer using a chemotherapy-free treatment approach of trastuzumab plus pertuzumab.

The breakthrough therapy designation was based on preliminary activity reported in the phase 2 RUN-HN clinical trial, which evaluated tipifarnib in patients with recurrent or metastatic HRAS-mutant head and neck squamous cell carcinoma.
A study published in The Lancet Hematology found an increased risk of developing myelodysplastic syndrome and acute myeloid leukemia when patients with cancer were treated with PARP inhibitors compared with placebo.

The chief of Gastrointestinal Radiation Oncology at the Rutgers Cancer Institute of New Jersey discussed how she hopes the results of the KEYNOTE-799 study will impact testing going forward.
Richard Schilsky, MD, touched on the research driving ASCO to award “Advance of the Year” to molecular profiling in gastrointestinal cancers, and what the organization’s top priorities are in the near future.

According to investigators on the CheckMate 9LA trial, these data support the use of nivolumab plus ipilimumab and 2 cycles of chemotherapy as a new first-line treatment for patients with advanced non–small cell lung cancer, regardless of PD-L1 expression or histology.