
Vismodegib is for the treatment of adults with metastatic basal cell carcinoma, or locally advanced basal cell carcinoma that has recurred following surgery or who are not candidates for surgery, and who are not candidates for radiation.

Your AI-Trained Oncology Knowledge Connection!


Vismodegib is for the treatment of adults with metastatic basal cell carcinoma, or locally advanced basal cell carcinoma that has recurred following surgery or who are not candidates for surgery, and who are not candidates for radiation.

Treatment of patients with locally advanced or metastatic non–small-cell lung cancer (NSCLC) that is anaplastic lymphoma kinase (ALK)-positive as detected by an FDA-approved test.

Vemurafenib is a protein kinase inhibitor that blocks the mutated gene protein BRAFV600E so that the cascade of messages that drive cell division and prevent apoptosis is stopped, resulting in tumor shrinkage.

Ipilimumab blocks cytotoxic T-lymphocyte antigen 4 (CTLA-4) and theoretically enables cytotoxic T cells to more effectively attack melanoma cells.
Advances in the multidisciplinary management of metastatic colorectal cancer have improved survival considerably, and nurses are key to optimal patient care.

Denosumab is an IgG2 monoclonal antibody that inhibits osteoclastic bone resorption (breakdown) via inhibition of RANKL (receptor activator of nuclear factor-kB ligand).

After IV administration, the human plasma protein binding of eribulin at concentrations of 100 ng/mL to 1,000 ng/mL ranges from 49% to 65%, and drug is eliminated (mean elimination half-life) in 40 hours.

Cabazitaxel is a microtubule inhibitor in the taxane class. It is made from a yew needle precursor using a semisynthetic process. It binds to free tubulin, which is used during mitosis to form two daughter cells.

Drug is indicated for the treatment of patients with cutaneous T-cell lymphoma (CTCL) who have received at least one prior systemic therapy.

Approved Drugs: Pazopanib (Votrient) Indications

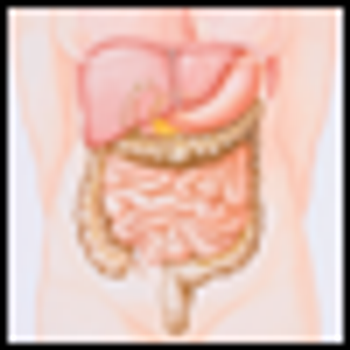
The colon, or large intestine, is the last part of the bowel where nutrients are absorbed. Stools are stored in the colon and excreted as a bowel movement.

El colon, o intestino grueso, es la última parte del intestino donde se absorben los nutrientes. Las heces se guardan en el colon y se excretan en forma de una evacuación intestinal.

None; investigational agents olaparib (AZD2281) and BSI-201 are in phase I and II clinical trials; other PARP inhibitors under investigation include AGO 14699 (Pfizer), ABT-888 (Enzo), and MK4827 (Merck).

In July 2009, the US Food and Drug Administration (FDA) granted approval for use of the vascular endothelial growth factor (VEGF) inhibitor bevacizumab (Avastin) in combination with interferon alfa for treatment of patients with metastatic renal cell carcinoma (RCC).

Once-daily oral inhibitor of mTOR (mammalian target of rapamycin) for the treatment of patients with advanced renal cell carcinoma (RCC) after failure of treatment with sunitinib (Sutent) or sorafenib (Nexavar).

Bendamustine HCl for injection is FDA approved for treatment of patients with chronic lymphocytic leukemia (CLL) or indolent B-cell non-Hodgkin’s lymphoma (NHL) that has progressed during or within 6 months of treatment with rituximab (Rituxan) or a rituximab-containing regimen.

Ixabepilone is indicated in combination with capecitabine (Xeloda) for the treatment of patients with metastatic or locally advanced breast cancer resistant to prior anthracycline and paclitaxel treatment (or for whom this treatment is contraindicated). Anthracycline resistance is defi ned as progression within 6 months of adjuvant therapy or 3 months of treatment for metastatic breast cancer; paclitaxel resistance is defi ned as progression on therapy or within 12 months of adjuvant therapy, or 4 months of treatment for metastatic breast cancer.

The cytochrome P450 microenzyme system has been an important protective system for living things for at least 3 billion years. It is a group or superfamily of isoenzymes that live in the endoplasmic reticulum and mitochondrial membrane of cells, and initially were responsible for detoxifying any poisons that were inhaled or ingested. As a result, these enzymes are found in the nose, saliva, kidneys, and lungs, and in greater numbers in the small intestines and liver. Cytochrome P450s account for about 75% of total metabolism and are important in oxidative metabolism-chemical modification/degradation of drugs.

Sorafenib is indicated for the treatment of patients with advanced renal cell cancer, and patients with unresectable hepatocellular cancer. Sunitinib is indicated for the treatment of patients with advanced renal cell cancer, and patients with gastrointestinal stromal tumor (GIST) after disease progression on imatinib mesylate (Gleevec).

Drug is indicated for rescue of normal cells following high dose methotrexate administration for osteosarcoma. It is also indicated to diminish and counteract methotrexate toxicity if the drug is not effectively eliminated, or for inadvertent overdose of folic acid antagonists.

Drug is a kinase inhibitor that blocks the action of mTOR (mammalian target of rapamycin). mTOR plays an important role in regulating key cellular functions, such as cell proliferation, survival, movement, and angiogenesis. It is part of the P13K (phosphoinositide 3-kinase)/Akt (protein kinase) signaling pathway which is often mutated in cancer. When mTOR is blocked, this leads to cell cycle arrest in the G1 phase of the cell cycle.

Ixabepilone is indicated in combination with capecitabine(Xeloda) for the treatment of patients with metastatic orlocally advanced breast cancer resistant to prior anthracyclineand paclitaxel treatment (or for whom this treatmentis contraindicated).

FDA-Approved Drugs: Vorinostat (Zolinza, suberoylanilide hydroxamic acid [SAHA]) Investigational Drugs: CRA-024781 (Celera Genomics), depsipeptide (Romidepsin)

FDA-Approved Drugs: 5-HT3 receptor antagonists Zofran (ondansetron), Kytril (granisetron), Anzamet (dolasetron), Aloxi (palonosetron); NK-1 receptor antagonist: Aprepitant (Emend)

Published: March 25th 2009 | Updated:

Published: February 19th 2010 | Updated:

Published: February 19th 2010 | Updated:

Published: February 16th 2011 | Updated:

Published: April 18th 2011 | Updated:

Published: February 1st 2008 | Updated: