
The study was evaluating whether a multicomponent intervention, compared with education alone, would result in a higher proportion of patients with ALL who have mercaptopurine adherence rates of 95% or higher.

Your AI-Trained Oncology Knowledge Connection!


The study was evaluating whether a multicomponent intervention, compared with education alone, would result in a higher proportion of patients with ALL who have mercaptopurine adherence rates of 95% or higher.
Researchers found that little of the mechanistic understanding of CIPN is currently being translated into clinical trials for interventions.

The platform was launched to provide cancer survivors live and on-demand workouts specifically geared towards survivors all types of cancers.

This study is intended to “investigate how chemotherapy disrupts sensory processing, memory, and attention in children; where in the brain the damage is occurring; and whether there is a biomarker that can identify those who are most vulnerable.”
Researchers suggested that potency enhancing phosphodiesterase-5 inhibitor drugs may have the ability to improve a prognosis in patients with colorectal cancer.
The combination was found to be safe and improved overall survival over azacitidine alone in certain patients with acute myeloid leukemia.
The recommendations were based on a review of 17 clinical trials, FDA approvals, and consensus where evidence was lacking.

The tablets were approved for the continued treatment of adult patients with acute myeloid leukemia who achieved first complete remission (CR) or CR with incomplete blood count recovery (CRi) following intensive induction chemotherapy and who are not able to complete intensive curative therapy.

Per the FDA, the new draft guidance represents an updated approach to clinical trial design and regulatory submissions.

The new drug application was based on data from a single, pivotal, randomized, controlled, phase 3 study of encequidar for the treatment of metastatic breast cancer.
The model was found to be superior to the United States Preventive Services Task Force (USPSTF) criteria at identifying African American ever-smokers for lung cancer screening.
Though further research is necessary, researchers suggested that immune checkpoint inhibitors can be used as an individualized therapy in certain patients who have undergone solid organ transplantation.

The new drug application is seeking approval for the treatment of adult patients with multiple myeloma whose disease is refractory to at least 1 proteasome inhibitor, 1 immunomodulatory agent, and 1 anti-CD-38 monoclonal antibody.
Researchers indicated that these findings suggest, “an ENE detection biomarker based on TP53 mutation detection would represent an enormous clinical benefit.”
With regard to the COVID-19 pandemic, researchers recommended that resection should occur as soon as possible depending on the availability of hospital resources and local disease burden.
The tool was developed “with the objective of providing transparency and facilitating surgical prioritization for treatment providers.”

This study found that abdominal or pelvic radiotherapy was associated with body composition changes that can adversely influence metabolic outcomes and performance status in survivors of abdominal or pelvic tumors.
Results from this trial observed in patients with RET-altered lung and thyroid cancers also led to the approval of selpercatinib by the FDA in May for this indication.
Researchers from Singapore discussed their own experiences and strategies used to safely manage breast cancer during the COVID-19 pandemic.
A phase 1b/2a study of the combination in patients with metastatic castration resistant prostate cancer demonstrated acceptable tolerability and potential efficacy.
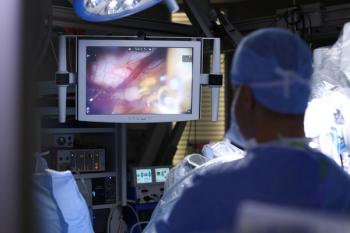
Researchers found that transoral robotic surgery “seems safe and effective compared to what’s been the standard of care for many years” for patients with early-stage oropharyngeal squamous cell carcinoma.

The FDA approved the FoundationOne Liquid CDx based on analytical and clinical validation studies that included more than 7500 samples and 30,000 unique variants across over 30 cancer types.
The updated guidelines now include tafasitamab-cxix in combination with lenalidomide with a category 2A designation as an option for the treatment of previously-treated adult patients with relapsed or refractory DLBCL not otherwise specified.
The recommendations were developed on the basis of the ESMO Scale for Clinical Actionability of molecular Targets (ESCAT) ranking for genomic alterations occurring in the 8 cancers responsible for the most deaths worldwide.

The study is evaluating asciminib in adult patients with Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase who were previously treated with 2 or more tyrosine-kinase inhibitors.

The new drug application was based on results from the pivotal ongoing, single-arm, phase 2 VISION study evaluating tepotinib monotherapy in patients with advanced NSCLC with METex14 skipping alterations.

The chief executive officer and co-founder of TrialJectory spoke about the online tool and what it offers for patients, providers, and pharmaceutical companies.

The study is evaluating enasidenib (Idhifa) plus best supportive care versus conventional care regimens in patients with relapsed or refractory acute myeloid leukemia with an isocitrate dehydrogenase-2 mutation.

The FDA approved a generic form of pemetrexed for injection as a single-agent in patients with locally advanced or metastatic non-squamous non-small cell lung cancer that has not progressed after 4 cycles of first-line platinum-based chemotherapy.

The application was based on results observed in the pivotal, phase 3 CheckMate-9ER trial evaluating cabozantinib in combination with nivolumab compared with sunitinib in previously untreated patients with advanced or metastatic RCC.