
“The importance of diverse representation cannot be underscored enough and is critical to ensure that safe and effective products are available to the [United States] patient population,” wrote the study authors, who were led by Nicole Gormley, MD.

Your AI-Trained Oncology Knowledge Connection!


“The importance of diverse representation cannot be underscored enough and is critical to ensure that safe and effective products are available to the [United States] patient population,” wrote the study authors, who were led by Nicole Gormley, MD.
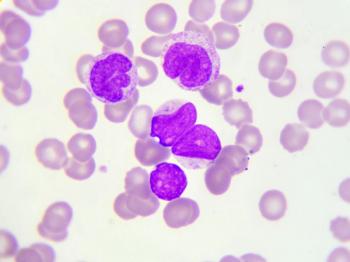
In this study, investigators sough to determine the incidence of CRLF2 rearrangements and IKZF1 deletions in Hispanic versus non-Hispanic children with B-cell acute lymphoblastic leukemia.
Given the current study findings, investigators suggested intratherapy personalization of chemoradiotherapy may possibly facilitate marked de-escalation of radiotherapy in patients with HPV-related oropharyngeal cancers.

In a phase 2 study, adavosertib plus gemcitabine showed signs of activity in platinum-resistant or platinum-refractory advanced high-grade serous ovarian cancer, including rare histological subtypes of ovarian cancer.
“Our results support further study of serial molecular imaging along with combined [histone deacetylase inhibitors; HDACi] and [aromatase inhibitor; AI] therapy (such as ECOG-ACRIN study E2112), to further delineate the role of HDACi and potential biomarkers in AI-refractory ER+ advanced breast cancer,” wrote the study authors.
“The [phase 1b/2] data [are] promising and support the current phase 3, multicenter trial, which we hope will lead to FDA approval and a new much-needed treatment option for this patient population,” said David Sallman, MD.
“Emerging evidence supports a possible role for overall dietary patterns that, in totality, emphasize habitually consuming fruits, vegetables, grains, and low-fat dairy and reducing red meat and alcohol intake,” wrote the study authors.
Given these study findings, investigators suggested that future efforts should address racial/ethnic, educational, financial, and geographic barriers to receiving digital breast tomosynthesis screening at the facility level.

“These updated results from the phase 2 MERECA trial underscore the positive impact on overall survival that ilixadencel may achieve for [patients with kidney cancer],” said Sven Rohmann, MD, PhD.
According to investigators, study findings could help guide treatment decisions and future research, as well as suggest new biomarkers for exploration.

The breakthrough therapy designation was based on preliminary activity reported in the phase 2 RUN-HN clinical trial, which evaluated tipifarnib in patients with recurrent or metastatic HRAS-mutant head and neck squamous cell carcinoma.

According to investigators on the CheckMate 9LA trial, these data support the use of nivolumab plus ipilimumab and 2 cycles of chemotherapy as a new first-line treatment for patients with advanced non–small cell lung cancer, regardless of PD-L1 expression or histology.

The results of a study on telehealth give insight into both the potential barriers and possible benefits of these services, which have been widely utilized since the start of the COVID-19 pandemic.

The sNDA submission for ruxolitinib to treat steroid-refractory chronic graft-versus-host disease in adult and pediatric patients 12 years and older was based on the phase 3 REACH3 study, which is assessing the safety and efficacy of ruxolitinib compared with best available therapy.

The approval of cemiplimab in non–small cell lung cancer was supported by results from the phase 3 EMPOWER-Lung 1 trial that investigated its use as monotherapy in the first-line setting compared with platinum-doublet chemotherapy in patients with locally advanced or metastatic disease whose tumor cells expressed PD-L1.
Continued approval for durvalumab in previously treated adult patients with locally advanced or metastatic bladder cancer was dependent upon results from the phase 3 DANUBE trial in the first-line metastatic bladder cancer setting, which did not meet its primary end points in 2020.
Investigators suggested that a new model could be used to identify service gaps at the population level after adjusting to the important local variations in cancer epidemiology.
“Patients from socially vulnerable communities had the most difficulty achieving a postoperative course without a complication, and they were the most likely to have an extended length of stay. These patients are in double and triple jeopardy,” said senior author Timothy Pawlik, MD.
These phase 3 study results support the use of sequential chemoradiation as a preferred adjuvant treatment following radical hysterectomy for patients with early-stage cervical cancer.
The medical oncologist and physician scientist based at the University of Texas MD Anderson Cancer Center explained recommendations for SARS-CoV-2 vaccination in patients participating in phase 1 oncology clinical trials.

The biologics license application is for the locally administered fusion protein Vicineum for the treatment of high-risk, bacillus Calmette-Guérin (BCG)–unresponsive non-muscle invasive bladder cancer.

In the OlympiA trial, patients with high-risk HER2-negative breast cancer were randomized 1:1 to receive either olaparib or placebo for 12 months.

A supplemental new drug application submission was primarily based on safety and efficacy data from the global phase 3 ASPEN trial of zanubrutinib compared with ibrutinib for the treatment of Waldenström macroglobulinemia.
A pooled analysis of 2 cohort studies suggested that individuals who began using aspirin before age 70 years and continued using the agent later in life had a reduced risk of colorectal cancer.

Building off of findings from the phase 3 NOVA study, this study revealed that an individualized starting dose of niraparib based on baseline bodyweight and platelet count could improve the tolerability of niraparib without affecting treatment outcomes.

Guidelines released by the Society for Immunotherapy of Cancer are intended to provide clinicians with the most current thinking on how experts can integrate immunotherapy into the treatment of patients with lymphoma.

The approval of trilaciclib was based on results from a pooled analysis of intent-to-treat datasets from 3 studies of patients with extensive-stage small cell lung cancer in which patients received standard chemotherapy plus either trilaciclib or placebo.

First-line treatment with nivolumab plus ipilimumab showed a moderate improvement in overall survival and a manageable safety profile in patients with metastatic uveal melanoma.

The FDA has granted breakthrough therapy designation to asciminib for the treatment of adult patients with Philadelphia chromosome–positive chronic myeloid leukemia in chronic phase who have been previously treated with 2 or more tyrosine kinase inhibitors; or who harbor the T315I mutation.
Per a planned interim superiority analysis conducted by an independent data monitoring committee, a trial of Eryaspace will continue without modification with final results anticipated later this year.

Published: October 20th 2020 | Updated:
Published: October 10th 2020 | Updated:
Published: August 24th 2020 | Updated:
Published: November 9th 2020 | Updated:

Published: October 26th 2020 | Updated:
Published: October 15th 2020 | Updated: