
The drug is currently the subject of an ongoing phase I/II clinical study for the treatment of patients with Ewing sarcoma who have relapsed or are refractory to standard-of-care therapy.

Your AI-Trained Oncology Knowledge Connection!


The drug is currently the subject of an ongoing phase I/II clinical study for the treatment of patients with Ewing sarcoma who have relapsed or are refractory to standard-of-care therapy.

The drug candidate tipifarnib (Zarnestra) is being clinically studied for the treatment of patients with HRAS-mutant head and neck squamous cell carcinoma after progression on platinum therapy.
True acupuncture resulted in fewer and less severe radiation-induced xerostomia symptoms in this phase III study.
Successfully eliminating Helicobacter pylori from a patient’s gastrointestinal tract could lead to a significant decrease in gastric cancer risk.

Mazyar Shadman, MD, MPH, discussed the study he and colleagues are conducting to evaluate CAR T-cell therapy in high-risk patients with chronic lymphocytic leukemia.
The ZUMA-II trial suggested that patients with relapsed/refractory mantle cell lymphoma resistant to prior therapies may benefit from the autologous anti-CD19 CAR T-cell therapy.

Stephen M. Hahn, MD, from The University of Texas MD Anderson Cancer Center, will take over as Commissioner of the FDA, voted on by the Senate on Thursday.
Molecular testing provided the clinical data and mutation profile of Chinese patients with colorectal cancer in this retrospective study.
Tanya Siddiqi, MD, discussed the CAR T-cell trial ahead of the ASH Annual Meeting & Exposition in an interview with CancerNetwork®.
Research suggests that there is an association between breast cancer risk and the use of chemical straighteners and permanent hair dyes.

The FDA approved FoundationOne CDx as a companion diagnostic for alpelisib in combination with fulvestrant for the treatment of men and postmenopausal women with HR+/HER2- PIK3CA-mutated breast cancer.
Additional positive interim safety and efficacy data were reported for annamycin in the treatment of patients with relapsed or refractory acute myeloid leukemia.
Patients who previously received radiotherapy for ductal carcinoma in situ may have higher mortality after developing an invasive second breast cancer.

The FDA has granted priority review to a supplemental biologics license application for pembrolizumab for the treatment of patients with BCG-unresponsive, high-risk, non-muscle invasive bladder cancer.
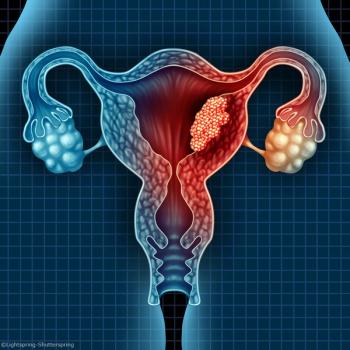
Patients with endometrial cancer pose a very high risk of dying from cardiovascular diseases in the year after diagnosis, suggesting the necessity of early involvement of cardiologists for these patients.
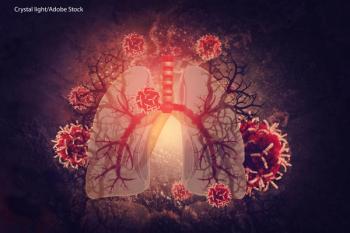
Takeda announced that their ongoing phase III ALTA-1L trial of brigatinib reduced the risk of disease progression or death in adults with advanced ALK-positive non-small cell lung cancer.
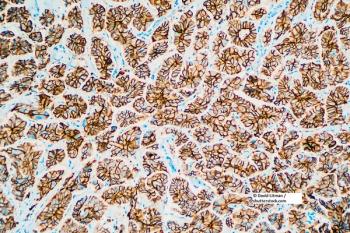
Henlius announced that their multicenter phase III study met its primary end point of best overall response rate at week 24.
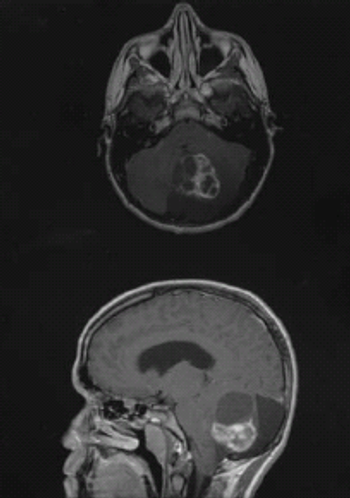
A perioperative study confirmed brain penetrance and robust biomarker suppression in patients with low-grade glioma with an IDH1 mutation.
Predictive models demonstrated the ability to anticipate adverse opioid-related outcomes among cancer survivors.

Researchers found that there is an increasing incidence of late stage head and neck cancer in the U.S., highlighting the need for continuous public health efforts toward early detection.
Researchers compared primary high grade serous ovarian carcinoma tumors and their matched metastasis, showing evidence of separating the 2 sample types and potentially predicting patient survival.

A new possible surveillance model suggests scanning patients with head and neck cancer treated with radiotherapy less frequently could be more cost-effective and time-effective.
Research suggests that oncogene S100A10 may play a definitive role in the progression of ovarian cancer and its expression levels may affect sensitivity to carboplatin.

Samsung Bioepis announced that the FDA accepted a biologics license application for SB8, a biosimilar candidate for bevacizumab.
MorphoSys announced that their ongoing phase III B-MIND study of tafasitamab passed the interim analysis for futility in patients with relapsed or refractory diffuse large B-cell lymphoma.

Oncotype DX® Breast Recurrence Scores from the National Cancer Database indicated that a lower threshold is needed for male patients with early stage ER-positive breast cancer to predict mortality.
Myovant announced that their phase III HERO study of relugolix met its primary efficacy end point and all 6 secondary endpoints in men with advanced prostate cancer.
Biomarker testing allows doctors to go beyond what’s under the microscope, helping to determine the best patient therapy.
An international study of prostate cancer clinical trials discovered that opioid use appears to differ by region, implying that a variability should be considered.

A quality improvement study indicated that nudges in the electronic health record were associated with a significant increase in clinician ordering of screening tests for breast and colorectal cancer.