Karyopharm Therapeutics announced plans to commence a global randomized clinical trial for low dose oral selinexor in hospitalized patients with severe COVID-19.

Your AI-Trained Oncology Knowledge Connection!


Karyopharm Therapeutics announced plans to commence a global randomized clinical trial for low dose oral selinexor in hospitalized patients with severe COVID-19.
This study found that raising the awareness of general practitioners and highlighting the importance of encouraging immigrant women to participate in cervical cancer screening is a feasible and effective strategy to increase participation in the program.
The expert oncologist spoke with CancerNetwork® about the impact that the COVID-19 pandemic has had on his practice and the impact it could have on medicine in general.
Representatives from multiple organizations convened to address the perceived variance among clinical practice guidelines for disease management in patients with cancer and patients with sickle cell disease.

Rutgers Cancer Institute of New Jersey will be conducting a clinical trial assessing whether azithromycin combined with hydroxychloroquine is better than hydroxychloroquine alone as a potential treatment for patients diagnosed with COVID-19.
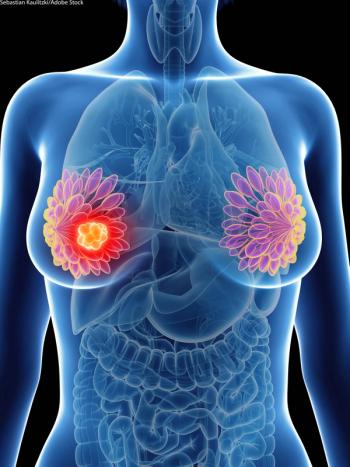
The decision to halt the study was based on the unanimous recommendation by an independent data safety monitoring committee during its routine review of the ASCENT study.

Leading cancer experts from across Europe issued an international expert consensus statement regarding radiotherapy treatment options for patients with rectal cancer during the COVID-19 pandemic.

The lymphoma expert, from the University of Nebraska Medical Center, spoke about how the COVID-19 pandemic has affected her facility and their ability to perform cancer care.
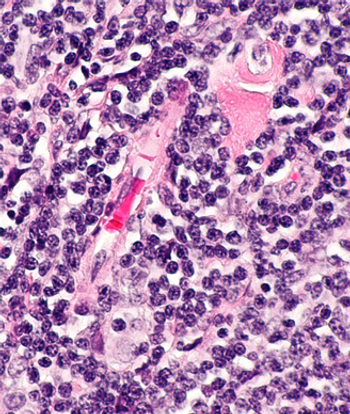
Michael L. Grossbard, MD, suggested that chemo-free regimens, including PI3K inhibitors and a more widespread use of allogeneic stem cell transplant, are being explored as treatment options in this space.
A case study of a patient in Wuhan, China demonstrated that tocilizumab may be an effective treatment for very ill patients with COVID-19 who also have multiple myeloma and other blood cancers.
CytoDyn announced that it has enrolled and treated the first 2 patients with COVID-19 with leronlimab under its phase II randomized clinical trial.
In a recent study, researchers indicated that primary liver cancer prevention schedules should give more attention to nonalcoholic steatohepatitis and elderly patients.
Providence Cancer Institute at Providence St. Joseph Health is pursuing a first-in-human phase I clinical trial of CORVax12, which is intended to act as a prophylactic vaccine to prevent COVID-19.

In an editorial, researchers discussed active and effective measures for the care of patients with cancer during the coronavirus disease 2019 (COVID-19) in China.

Despite positive trends, researchers found that substantial differences in the representation of gender in oncology publications by author role, article type, citation count, and credentials persist.

Incyte announced that they are working with the FDA to initiate a phase III clinical trial to assess ruxolitinib (Jakafi) plus standard-of-care in patients with coronavirus disease 2019 (COVID-19) induced cytokine storm.

The FDA granted priority review to pembrolizumab monotherapy for the treatment of adult and pediatric patients with unresectable or metastatic TMB-high solid tumors who have progressed following prior treatment and who have no satisfactory alternative treatment options.
Researchers found that in patients with chronic lymphocytic leukemia receiving commercial ibrutinib, initial dose and dose modification during therapy did not appear to impact event-free survival or overall survival.
A 1-year follow-up of the phase II ZUMA-2 study found that KTE-X19 induced durable remissions in a majority of patients with relapsed or refractory mantle-cell lymphoma.

The director of the National Institute of Allergy and Infectious Diseases spoke with The New England Journal of Medicine in a podcast about how physicians can approach talking to patients regarding COVID-19.

Two separate studies of pediatric patients in Wuhan, China found that though clinical manifestations of COVID-19 in children were less severe than those of adult patients, young children, particularly infants, were vulnerable to the infection.

The FDA granted fast track designation to ME-401, an investigational selective oral inhibitor of PI3K delta, for the treatment of adult patients with relapsed or refractory follicular lymphoma.
In a letter to Congress, the AACR called for immediate action to be taken to protect patients with cancer against the COVID-19 pandemic.

The FDA cleared the investigational new drug (IND) application for the use of CYNK-001 in adults with COVID-19.
The trial evaluating the first-line treatment of pembrolizumab in patients with microsatellite instability-high or mismatch repair deficient unresectable or metastatic colorectal cancer demonstrated improved PFS compared with chemotherapy.

In a webinar hosted by the American Nurses Association, Terri Rebmann, PhD, RN, CIC, FAPIC, discussed how nurses can protect themselves and their patients during the COVID-19 pandemic.

Clinicians advised the best approaches for treating patients who have been diagnosed or are currently under treatment for cancer during the ongoing COVID-19 pandemic.
Researchers reported updated findings of infigratinib in upper tract urothelial carcinoma and urothelial carcinoma of the bladder, supporting the development of phase III studies in this setting.
Immutep reported positive data from the randomized phase IIb AIPAC clinical trial of eftilagimod alpha and paclitaxel in HER2-negative, HR-positive metastatic breast cancer (MBC).

In a podcast, published by The New England Journal of Medicine (NEJM), physicians discuss practical measures that could help prevent the coronavirus disease 2019 (COVID-19).