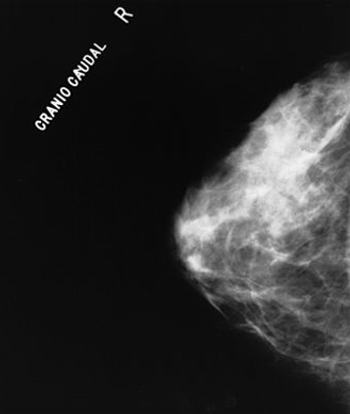
A Swedish study found that 3D tomosynthesis screening detected 40% more cases of breast cancer compared with traditional digital mammography.

Your AI-Trained Oncology Knowledge Connection!


A Swedish study found that 3D tomosynthesis screening detected 40% more cases of breast cancer compared with traditional digital mammography.

The appearance of bone metastases on FDG PET/CT was influenced by breast cancer histology, with non-FDG-avid lesions more common in invasive lobular carcinoma patients.

In a mouse model, researchers found that TRAIL-R2 inhibition prevented bone metastases, suggesting a possible therapeutic strategy for breast cancer patients.
A small retrospective study of heavily pretreated patients with chronic myeloid leukemia found bosutinib to be a good option in the fourth-line setting.

The risk of bone metastases from GISTs, though rare, should be considered during long-term follow-up of patients, especially in those with liver metastases.

The US Food and Drug Administration has approved dinutuximab (Unituxin) for the treatment of high-risk neuroblastoma in pediatric patients.

The US Food and Drug Administration has approved filgrastim-sndz (Zarxio), a biosimilar of filgrastim (Neupogen), and the first such agent to be approved in the United States.

The FDA has approved nivolumab (Opdivo) for the treatment of metastatic squamous non-small-cell lung cancer (NSCLC) in patients who have progressed on a platinum-based chemotherapy.

The FDA has approved panobinostat (Farydak), in combination with bortezomib and dexamethasone, for treating patients with multiple myeloma.

A recent study found that radiation therapy was effective for the palliation of painful spinal metastases in patients with hepatocellular carcinoma.

The FDA has approved lenvatinib (Lenvima) for the treatment of patients with progressive, differentiated thyroid cancer refractory to radioactive iodine.

The US Food and Drug Administration has granted Orphan Drug Designation to pelareorep (Reolysin) for the treatment of ovarian cancer.

The FDA has approved the CDK4/6 inhibitor palbociclib (Ibrance) for the treatment of postmenopausal women with metastatic breast cancer.

A urine test (CellDetect) was able to detect disease recurrence in bladder cancer patients, with a reported sensitivity of 84.4% and specificity of 82.7%.

The FDA has expanded the approved use of ibrutinib (Imbruvica) to include patients with Waldenström macroglobulinemia, a rare type of non-Hodgkin lymphoma.

The US Food and Drug Administration has granted orphan drug designation to tarextumab for the treatment of pancreatic cancer and small-cell lung cancer.

The FDA has approved blinatumomab (Blincyto) for the treatment of patients with Philadelphia chromosome-negative precursor B-cell acute lymphoblastic leukemia.

The FDA has approved combined netupitant and palonosetron (Akynzeo) for the treatment of chemotherapy-related nausea and vomiting in patients with cancer.

The FDA granted accelerated approval to pembrolizumab (Keytruda) for treating patients with advanced melanoma who are no longer responding to other drugs.

The US Food and Drug Administration has approved bevacizumab (Avastin) for the treatment of patients with recurrent or metastatic cervical cancer.

The FDA has approved the drug belinostat to treat patients with relapsed or refractory peripheral T-cell lymphoma.

Earlier today the FDA granted accelerated approval to ceritinib (Zykadia) for the treatment of patients with metastatic ALK-positive non-small-cell lung cancer (NSCLC).

The FDA has approved siltuximab (Sylvant) for the treatment of multicentric Castleman disease, a disorder of the lymph nodes that is similar to lymphoma and often treated with chemotherapy and radiation.

The FDA has approved the angiogenesis inhibitor ramucirumab for the treatment of gastric cancer and gastroesophageal junction adenocarcinoma.

The FDA has approved an HPV DNA test to be used as a primary screening method for cervical cancer in women 25 and older. The test can also give insight into future risk of cervical cancer.

The FDA has approved ibrutinib (Imbruvica) for the treatment of patients with mantle-cell lymphoma (MCL), an aggressive and rare blood cancer.

The FDA today approved obinutuzumab (Gazyva) for the treatment of chronic lymphocytic leukemia, to be used in combination with chlorambucil.

The US Food and Drug Administration approved the multi-kinase inhibitor regorafenib (Stivarga) yesterday, for the treatment of patients with unresectable metastatic gastrointestinal stromal tumors (GIST) that no longer respond to imatinib and sunitinib.

The FDA has approved ado-trastuzumab emtansine (Kadcyla), known as T-DM1 in development, for the treatment of women with metastatic HER2-positive breast cancer.

The FDA has approved pomalidomide (Pomalyst) to treat patients with relapsed or refractory multiple myeloma who have received at least two prior therapies.