
The US Food and Drug Administration has approved imatinib (Gleevec) for the treatment of newly diagnosed Philadelphia chromosome–positive acute lymphoblastic leukemia (Ph+ ALL) in children.

Your AI-Trained Oncology Knowledge Connection!


The US Food and Drug Administration has approved imatinib (Gleevec) for the treatment of newly diagnosed Philadelphia chromosome–positive acute lymphoblastic leukemia (Ph+ ALL) in children.

The US Food and Drug Administration has approved ponatinib (Iclusig) to treat adults with chronic myeloid leukemia and Philadelphia chromosome-positive acute lymphoblastic leukemia.

The expanded FDA approval of abiraterone acetate (Zytiga) now includes its use prior to chemotherapy in men with metastatic castration-resistant prostate cancer.

The US Food and Drug Administration (FDA) has approved cabozantinib (Cometriq) for the treatment of metastatic medullary thyroid cancer, a rare type of thyroid cancer that makes up about 4% of all cases.

The FDA today approved omacetaxine mepesuccinate (Synribo) for the treatment of adults with chronic myeloid leukemia (CML) who are resistant to other therapies.

AVEO Pharmaceuticals Inc has announced that it has submitted a new drug application for tivozanib to the US Food and Drug Administration for the treatment of metastatic renal cell carcinoma.
Results from a phase Ib/II trial presented at the ESMO 2012 Congress found that a tumor-targeting doxorubicin conjugate, aldoxorubicin (INNO-206), showed activity in relapsed soft-tissue sarcoma patients.

The FDA has approved the multikinase inhibitor regorafenib (Stivarga) to treat patients with colorectal cancer that has metastasized following previous treatment.

The FDA has approved an ultrasound device shown to be capable of detecting small masses in dense breasts. The device is indicated for use in combination with mammography for breast cancer screening.

The FDA has approved the production and use of 11C choline, an agent used to detect recurrent prostate cancer during PET imaging.

A new study found that aspirin use was associated with a reduced risk of prostate cancer mortality in patients previously treated with prostatectomy or radiotherapy.

The FDA approved the tyrosine kinase inhibitor bosutinib (Bosulif) to treat Philadelphia chromosome–positive chronic myelogenous leukemia (CML).

The FDA has approved tbo-filgrastim, a drug that reduces the time that cancer patients with non-myeloid malignancies experience severe chemo-related neutropenia.

The FDA approved a dissolvable form of everolimus (Afinitor Disperz) for the treatment of children with subependymal giant cell astrocytoma (SEGA) that cannot be treated with surgery.

The FDA has approved vincristine sulfate liposome injection (Marqibo) to treat adults with Philadelphia chromosome–negative acute lymphoblastic leukemia (ALL).

According to a study in the journal Cancer, without the use of PSA screening the number of men presenting with cases of metastatic prostate cancer would be three times greater than the actual number observed today.

The FDA has approved aflibercept (Zaltrap) to be used with the chemotherapy regimen FOLFIRI in the treatment of adults with metastatic colorectal cancer.

Last week the US Food and Drug Administration approved carfilzomib (Kyprolis) to treat patients with multiple myeloma who have received at least two prior therapies, including treatment with bortezomib (Velcade) and an immunomodulatory therapy.

Earlier today the FDA approved pazopanib (Votrient) to treat patients with advanced soft-tissue sarcoma who have previously received chemotherapy. More than 20 subtypes of sarcoma were included in the clinical trial that led to the approval.

The FDA has granted imatinib full approval as an adjuvant treatment following surgical removal of CD117-positive gastrointestinal stromal tumors in adult patients. This comes after results from a phase III trial showed that patients taking imatinib for 36 months had a 5-year overall survival of 92%, compared to 82% for those patients who took the drug for the standard 12 months of treatment.

The US Food and Drug Administration (FDA) announced the approval of vismodegib (Erivedge), for the treatment of advanced basal cell carcinoma, the most common type of skin cancer, for patients who are not eligible for surgery or radiation, and for metastatic disease.

The US Food and Drug Administration (FDA) has approved the angiogenesis blocker axitinib (Inlyta), a twice daily oral drug, as a second-line treatment for patients with advanced renal cell carcinoma.

The US Food and Drug Administration has approved asparaginase Erwinia chrysanthemi for the treatment of patients with acute lymphoblastic leukemia, who have developed hypersensitivity to E. coli derived asparaginase and pegaspargase chemotherapy.

The US Food and Drug Administration announced today that it has revoked the approval of bevacizumab for breast cancer due to the potentially life-threatening side effects associated with the treatment. It was approved for metastatic breast cancer in February 2008, but data later showed that along with an increase in side effects, there was no increase in overall survival.

The U.S. Food and Drug Administration (FDA) has approved two new indications for the osteoporosis drug denosumab, as a treatment for bone loss in men receiving androgen deprivation therapy for nonmetastatic prostate cancer and in women receiving adjuvant aromatase inhibitor therapy for breast cancer.
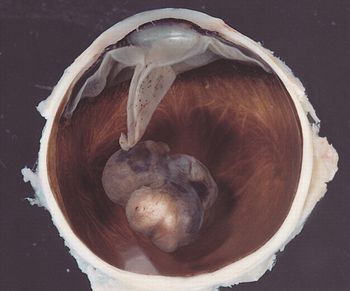
Researchers have discovered that individuals carrying a mutation in the BAP1 gene are at greater risk of developing mesothelioma and uveal melanoma.

The FDA has approved crizotinib (Xalkori) for the treatment of late-stage non–small-cell lung cancer patients who express the abnormal anaplastic lymphoma kinase (ALK) gene.

The U.S. Food and Drug Administration (FDA) announced last week the approval of brentuximab vedotin, a CD30-directed antibody drug-conjugate, for the treatment of refractory Hodgkin lymphoma and systemic anaplastic large-cell lymphoma.

The U.S. Food and Drug Administration approved vemurafenib (Zelboraf) for the treatment of metastatic or unresectable melanoma. The new drug, also known as PLX4032, specifically targets patients whose tumors express the BRAF V600E gene mutation.

The picture in 2011 for the community oncologist is dire. Over the past several years many community practices have been acquired by hospitals out of duress. Drug delivery has started to change as treatments are evolving, moving away from the high-margin infusion business.