
The US Food and Drug Administration has approved abiraterone acetate (Zytiga) tablets in combination with prednisone for the treatment of metastatic prostate cancer patients with high-risk, castration-sensitive disease.

Your AI-Trained Oncology Knowledge Connection!


The US Food and Drug Administration has approved abiraterone acetate (Zytiga) tablets in combination with prednisone for the treatment of metastatic prostate cancer patients with high-risk, castration-sensitive disease.

The FDA has expanded the approval of afatinib (Gilotrif) to include the first-line treatment of metastatic non–small-cell lung cancer patients whose tumors have non-resistant EGFR mutations, including three newly identified substitution mutations.

The FDA has expanded the approval of the PARP inhibitor olaparib (Lynparza) to include the treatment of patients with metastatic breast cancer who have a mutated BRCA gene.

The FDA has approved brentuximab vedotin (Adcetris) for the treatment of primary cutaneous anaplastic large-cell lymphoma and CD30-expressing mycosis fungoides in patients who have received prior systemic therapy.

The FDA has expanded the approval of vemurafenib (Zelboraf) to include the treatment of Erdheim–Chester disease in adult BRAF V600–positive patients. This marks the first approval from the agency for treating this rare blood cancer.

The FDA has granted accelerated approval to acalabrutinib (Calquence) for the treatment of mantle cell lymphoma in adult patients who have received at least one previous therapy.

The FDA has approved the CAR T-cell therapy axicabtagene ciloleucel (Yescarta) for the treatment of large B-cell lymphomas in adults who have failed or relapsed after two or more prior treatments.

The FDA has granted accelerated approval to nivolumab (Opdivo) for the treatment of patients with hepatocellular carcinoma who have previously received sorafenib.

The FDA has approved gemtuzumab ozogamicin (Mylotarg) for the first-line treatment of adults with CD33-positive AML and for pediatric patients with relapsed or refractory CD33-positive AML.

The FDA has approved the novel PI3K inhibitor copanlisib (Aliqopa) for the treatment of relapsed follicular lymphoma in adult patients who have received at least two prior systemic therapies.

The FDA has approved inotuzumab ozogamicin (Besponsa) for the treatment of adults with relapsed or refractory B-cell precursor acute lymphoblastic leukemia.

Women with increased levels of cadmium, which mimics estrogen in the body, had a higher risk of endometrial cancer, according to a recently published observational study.

The FDA has approved a fixed combination of daunorubicin and cytarabine (Vyxeos) for the treatment of newly diagnosed therapy-related acute myeloid leukemia (AML) as well as AML with myelodysplasia-related changes.

The FDA has approved enasidenib (Idhifa) for the treatment of relapsed or refractory IDH2-mutant acute myeloid leukemia.

The FDA Oncologic Drugs Advisory Committee has unanimously voted to recommend approval of the trastuzumab biosimilar MYL-1401O for the treatment of HER2-positive breast cancer.

The FDA has expanded the approval of ipilimumab (Yervoy) to include the treatment of pediatric melanoma patients 12 years and older with unresectable or metastatic disease.

The FDA has expanded the approval of blinatumomab (Blincyto) for the treatment of relapsed or refractory B-cell precursor acute lymphoblastic leukemia in adults and children.

The FDA has granted approval to pembrolizumab for pediatric and adult patients with microsatellite instability (MSI)-high or mismatch repair (MMR)-deficient solid tumors.

The FDA has granted approval to pembrolizumab (Keytruda) in the first- and second-line settings for the treatment of patients with locally advanced or metastatic urothelial carcinoma.

The FDA has approval pembrolizumab (Keytruda), in combination with chemotherapy, for the first-line treatment of patients with metastatic non-squamous non–small-cell lung cancer.

The FDA has granted accelerated approval to avelumab (Bavencio) for treating locally advanced or metastatic urothelial carcinoma patients whose disease progressed following treatment with platinum-containing chemotherapy.

The FDA has approved durvalumab (Imfinzi) for the treatment of patients with advanced urothelial carcinoma whose disease has progressed after treatment with platinum-containing chemotherapy.

The FDA has approved regorafenib (Stivarga) for the second-line treatment of hepatocellular carcinoma (HCC) for patients who have previously received sorafenib.

Interim data from CheckMate 040 showed that nivolumab produces durable responses with promising long-term survival rates in patients with advanced, unresectable hepatocellular carcinoma.
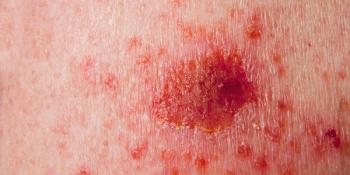
Treatment of keratinocyte carcinomas such as basal cell carcinoma and cutaneous squamous cell carcinoma with PD-1 checkpoint inhibitors and targeted agents warrant further investigation, according to recently published studies.

The FDA has approved telotristat ethyl (Xermelo) tablets for the treatment of patients with carcinoid syndrome diarrhea that has not responded to somatostatin analogs alone.

The FDA has expanded the approval of lenalidomide to include its use as maintenance therapy for patients with multiple myeloma following autologous stem cell transplant.

Long-term data from the phase III BC2001 trial confirmed that adding chemotherapy to radiation therapy improves locoregional control and reduces the rate of salvage cystectomy in patients with muscle-invasive bladder cancer.

Tailored dose-dense chemotherapy in high-risk early breast cancer patients did not show a significant improvement in recurrence-free survival compared with standard adjuvant chemotherapy.

Results from a phase I/II study suggest that the immunotherapy nivolumab is safe and effective in advanced hepatocellular carcinoma (HCC).

Published: September 11th 2012 | Updated:
Published: October 2nd 2012 | Updated:

Published: October 26th 2012 | Updated:

Published: December 11th 2012 | Updated:

Published: December 14th 2012 | Updated:

Published: February 11th 2013 | Updated: