Hematologic Oncology
Latest News
Latest Videos

CME Content
More News
Continuing their focus on optimizing JAK inhibitor therapy in myelofibrosis, panelists highlight dosing and adverse event management strategies.
A brief discussion on the role that JAK inhibitors play in cytopenic myelofibrosis and how best to optimize care with sequencing and dose adjustment.

Data from a phase 2 trial support a supplemental new drug application for isavuconazonium sulfate as a treatment for pediatric patients with invasive aspergillosis or invasive mucormycosis.

A broader overview of the role that JAK inhibitors play in patients with myelofibrosis and how that role has continued to evolve in the current treatment paradigm.
Moving on to review the first patient scenario of primary myelofibrosis, panelists elucidate the decisionmaking process when selecting JAK inhibitor therapy.

Expert panelists share brief insight on the current NCCN guidelines for selecting treatment in patients with myelofibrosis.

Focused discussion on the factors that help to select patients for stem cell transplantation over systemic therapy in the setting of myelofibrosis.
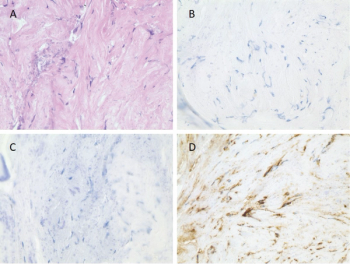
Investigators report a case of a man, aged 55 years, with an extensive and prolonged course of an unexplained multi-systemic disease, and also review common clinical manifestations, mutations, diagnoses, and targeted therapies for Erdheim-Chester disease.

The difference in utilizing cytomegalovirus reactivation outcomes while on posttransplantation cyclophosphamide vs other graft-vs-hose disease agents has led to a better understanding in long-term disease and reinfection rates.

A recent study has found that novel graft-versus-leukemia has minor histocompatibility antigens and was all validated.

A comprehensive discussion on the respective role stem cell transplantation has in the treatment armamentarium for myelofibrosis.

Key opinion leaders in myelofibrosis management reflect on the role of biomarkers in informing treatment selection, even for patients with triple-negative disease.

Data from the phase 2 ROCKstar trial support the approval of belumosudil as a treatment for patients with chronic graft-versus-host-disease in Scotland.

Patients with select hematologic cancers, including lymphoma and multiple myeloma, appear to benefit from a ready-to-dilute formation of intravenous cyclophosphamide, the new drug application for which was approved by the FDA.

An experimental cyclophosphamide-based prophylaxis regimen may also elicit a lower rate of chronic graft-versus-host-disease vs standard prophylaxis in patients with hematologic cancers.

A retrospective analysis of more than 17,000 patients with hematologic malignancies identified a difference in median survival between patients who received palliative care in addition to hematopoietic stem cell transplantation and those who didn’t.
A real-world population of patients with relapsed/refractory B-cell acute lymphoblastic leukemia are reported to have had a high rate of complete remissions following treatment with brexucabtagene autoleucel.
Obecatagene autoleucel also appears to result in a high rate of minimal residual disease negativity in a population of patients with relapsed/refractory B-cell acute lymphoblastic leukemia.

A recently published article highlights the use of CD4-positive/CD8-positive double-positive T cells as a predictive marker for graft-versus-host disease.

Adult patients with indolent systemic mastocytosis can now receive avapritinib following the FDA’s approval of the agent.

Findings from the phase 2 MAJIC-PV trial highlight a superior event-free survival and overall survival following ruxolitinib vs best available treatment in patients with polycythemia vera who were intolerant/resistant to hydroxycarbamide.

Lead study author Ruben Mesa, MD, discusses findings from the MOMENTUM trial assessing the use of momelotinib in the treatment of myelofibrosis.
In older patients with hematologic malignancies undergoing hematopoietic stem cell transplantation, psychological distress at baseline was associated with worse quality of life outcomes.

A recent study found for patients with graft-versus-host disease, a relationship was observed between CD38 expression which reduced survival and had a higher transplant-related mortality.

Corey Cutler, MD, MPH, FRCPC, discusses how patients with blood cancer in need of an umbilical cord blood transplant may benefit from omidubicel-onlv.