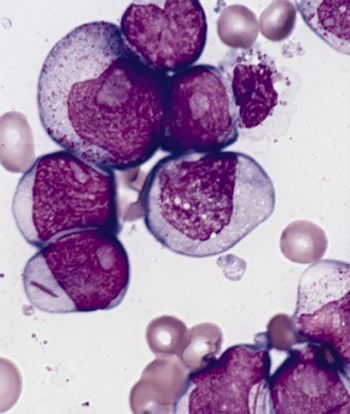
Investigators say that a phase 1/2 trial evaluating MGTA-117 in 2 hematologic malignancies has been paused following a serious adverse effect that may be related to the agent.

Your AI-Trained Oncology Knowledge Connection!


Investigators say that a phase 1/2 trial evaluating MGTA-117 in 2 hematologic malignancies has been paused following a serious adverse effect that may be related to the agent.

“Nonetheless, we saw tremendous benefits for these patients, patients who didn’t benefit from other treatments, and it was exciting to be involved with this early research.”

Combining bispecific antibodies with other agents such as R-CHOP and R-CHP for various subtypes of lymphoma has the potential to produce exciting results, according to an expert from Dana-Farber Cancer Institute.
Richard L. Martin III, MD, MPH, and Stephen Schleicher, MD, MBA, share a perspective on rural cancer care.
Early study results show that an artificial intelligence model may help oncologists better stratify patients with prostate cancer into risk groups than existing methods, according to an expert.

Adding radiation to sorafenib elicited a survival improvement in a group of patients with hepatocellular carcinoma, a type of liver cancer.
Minh-Tri Nguyen, MD, and colleagues investigate the association between time to treatment, socioeconomic status, and clinical outcomes among rural and urban patients with breast cancer.

Patients with estrogen receptor–positive, HER2-negative metastatic breast cancer can now receive elacestrant as treatment following the FDA’s approval of the agent.

The approval for pirtobrutinib in the treatment of patients with relapsed or refractory mantle cell lymphoma was based on data from the phase 1/2 BRUIN trial.

The use of circulating tumor DNA in patients with advanced colorectal cancer may provide early insight into who is and is not responding to treatment, according to an expert from Weill Cornell Medicine.
Qianyue Deng, MD, and colleagues examine the efficacy of thoracic radiotherapy for the treatment of unresectable non-small cell lung cancer.

The European Commission approval for trastuzumab deruxtecan in HER2-low metastatic breast cancer is based on data from the phase 3 DESTINY-Breast04 trial.
Adjuvant chemotherapy following multiagent neoadjuvant chemotherapy and surgical resection produced better overall survival among patients with pancreatic ductal adenocarcinoma vs those who did not receive adjuvant treatment.

The FDA approved adjuvant pembrolizumab for the treatment of patients with resected non–small cell lung cancer, based on data from the phase 3 KEYNOTE-091/EORTC-1416-LCG/ETOP-8-15-PEARLS trial.
Tamibarotene is a first-in-class oral first-in-class selective retinoic acid receptor α agonist that is being evaluated in combination with azacitidine for newly diagnosed higher-risk myelodysplastic syndrome.

Black and Hispanic patients are less likely to receive opioids than their White counterparts; bias training and logistical support may act as potential strategies to mitigate these disparities, according to an expert from Dana-Farber Cancer Institute.

Treatment with avutometinib and defactinib in patients with low-grade serous ovarian cancer resulted in a positive objective response rate.
Co-editor-in-Chief Howard S. Hochster, MD, discusses disparities in access to health care between urban and rural patients with breast cancer, and how they might be addressed.

Data from a ctDNA analysis of the phase 3 INTRIGUE study indicate that KIT mutational status may be associated with response to certain Tyrosine kinase inhibitors in GIST, according to an expert from the Yale Cancer Center in New Haven, Massachusetts.

An expert from the Smilow Cancer Hospital and Yale Cancer Center indicates that findings from the ctDNA analysis of the phase 3 INTRIGUE study were “provocative” and that the subsequent phase 3 INSIGHT study has the potential to be practice changing should data be positive.

Findings from a phase 2 trial indicate that CTX-009 plus paclitaxel may produce an encouraging responses in patients with advanced biliary tract cancer, according to investigators.

Neoadjuvant pembrolizumab appears to improve outcomes in a cohort of patients with metastatic microsatellite instability–high, mismatch repair deficient solid tumors.

Trial results presented at a recent medical conference demonstrated that the use of shorter duration radiation is safe and effective in prostate cancer, as well as a type of rare cancer, according to an expert.

Treatment with nivolumab plus ipilimumab appears to result in overall survival and progression-free survival benefit in patients with intermediate- or poor-risk sarcomatoid renal cell carcinoma.

DB-1303, an investigational third generation antibody-drug conjugate that now has FDA fast track designation, may benefit patients with HER2-overexpressing endometrial cancer.
Data from the phase 2 PIONEER trial supports the supplemental new drug application for avapritinib in adult patients with indolent systemic mastocytosis, an uncontrolled proliferation and activation of mast cells.

Findings from the phase 3 BREAKWATER study indicate that encorafenib plus cetuximab and chemotherapy produces activity and is well-tolerated in metastatic colorectal cancer harboring BRAF V600E mutations.

Findings from the phase 2 MOUNTAINEER trial indicate that HER2 scoring algorithms may help in identifying which patients are suitable to receive tucatinib plus trastuzumab for metastatic colorectal cancer.

Data presented at a recent medical conference shows that there is continued interest in observing the effects of fewer radiation treatments across several cancer types, according to an expert.
Findings from the phase 3 SUNLIGHT study highlight favorable overall survival and disease control rates with the use of bevacizumab plus trifluridine and tipiracil in metastatic colorectal cancer.